Phillip Taud
Member
- Joined
- Jan 4, 2019
- Messages
- 14
- Reaction score
- 1
Hi there,
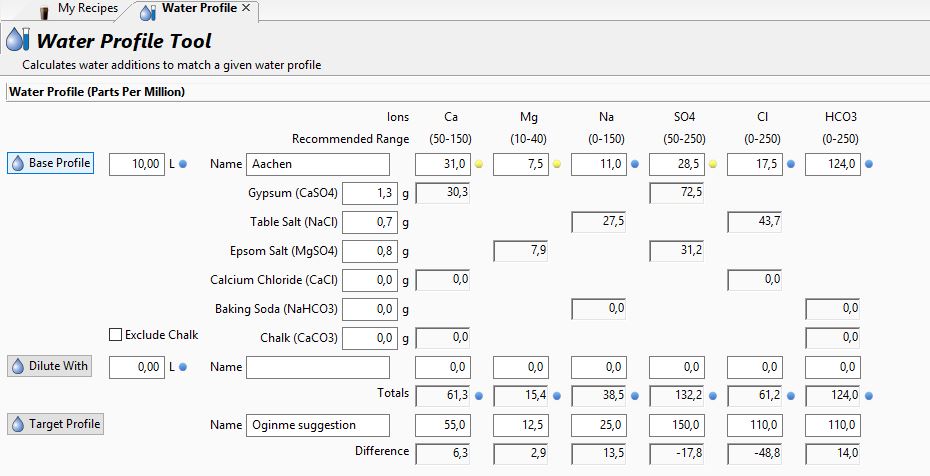
I am here in Germany trying to brew crisp, dry Pale Ales / IPAs and fresh american Lagers. So far I have been aiming for these water targets. What are your thoughts and am I doing the right thing? My beers definitely could be dryer. My pre-mash pH is also usually a little high at 5,9 which I have not adjusted so far.
Suggestions would be great!
Thx, Phillip
I am here in Germany trying to brew crisp, dry Pale Ales / IPAs and fresh american Lagers. So far I have been aiming for these water targets. What are your thoughts and am I doing the right thing? My beers definitely could be dryer. My pre-mash pH is also usually a little high at 5,9 which I have not adjusted so far.
Suggestions would be great!
Thx, Phillip






























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)




























