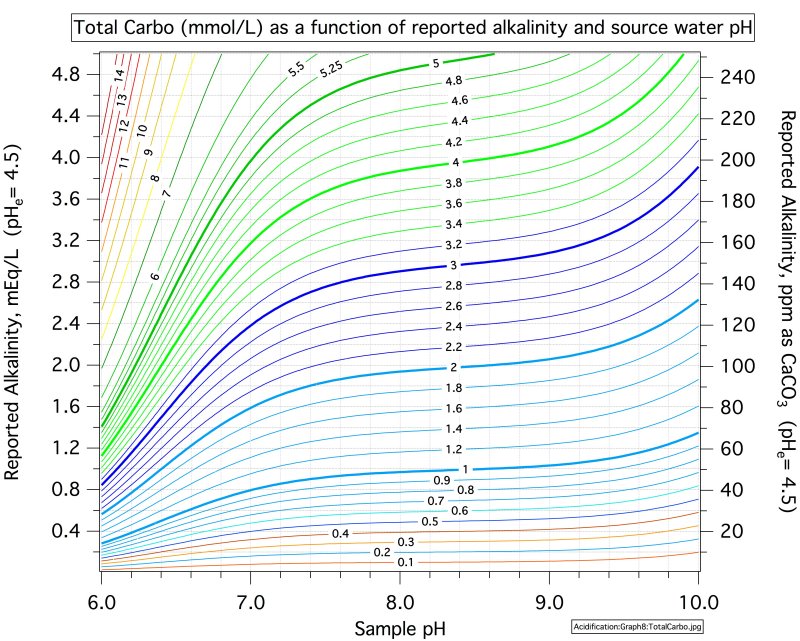
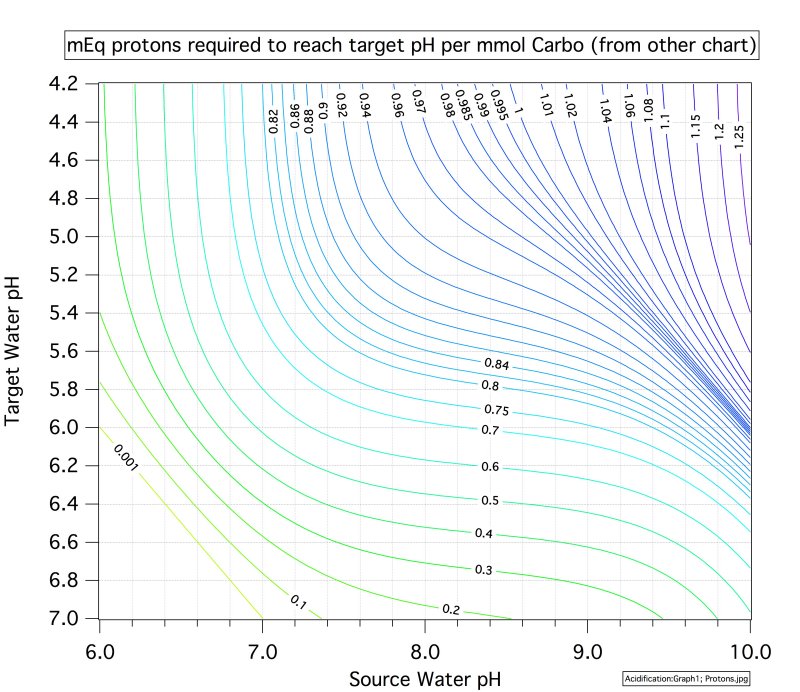
One of my favorite beers is Oatmeal Stout. My recipe uses 5% Crystal Malt and 7% roasted UK malts. My new brewing location has different water chemistry, and my opinion is that the beer is not quite as good as it was. On the last batch, brewed this weekend, I was planning to use CaC03, but I read up a bit on its use, and changed my plans. Instead, I decided to monitor the mash pH and add nothing.
The mash pH started and ended at about 4.9.
The flavor difference, and reason I care, is that the new water doesn't seem to produce the rich, chocolatey flavor that the original beer had. The roastiness comes through (yeah, it ought to) but it has a somewhat hollow character and tastes a bit more burnt than browned. I never tested the pH of my mash at the old place.
What should I do to get my pH back in line?
***NEW (municipal) WATER*** Ward Labs Data:
Just for comparison...
***OLD (well) WATER*** Ward Labs Data:
The mash pH started and ended at about 4.9.
The flavor difference, and reason I care, is that the new water doesn't seem to produce the rich, chocolatey flavor that the original beer had. The roastiness comes through (yeah, it ought to) but it has a somewhat hollow character and tastes a bit more burnt than browned. I never tested the pH of my mash at the old place.
What should I do to get my pH back in line?
***NEW (municipal) WATER*** Ward Labs Data:
Code:
pH 8.0
Total Dissolved Solids (TDS) Est, ppm 121
Electrical Conductivity, mmho/cm 0.20
Cations / Anions, me/L 1.8 / 1.7
ppm
Sodium, Na 24
Potassium, K 3
Calcium, Ca 8
Magnesium, Mg 3
Total Hardness, CaCO3 33
Nitrate, NO3-N 0.4 (SAFE)
Sulfate, SO4-S 11
Chloride, Cl 11
Carbonate, CO3 < 1.0
Bicarbonate, HCO3 37
Total Alkalinity, CaCO3 31
Total Phosphorus, P 0.25
Total Iron, Fe < 0.01Just for comparison...
***OLD (well) WATER*** Ward Labs Data:
Code:
pH 7.7
Total Dissolved Solids (TDS) Est, ppm 249
Electrical Conductivity, mmho/cm 0.41
Cations / Anions, me/L 4.6 / 4.5
ppm
Sodium, Na 10
Potassium, K 2
Calcium, Ca 61
Magnesium, Mg 12
Total Hardness, CaCO3 203
Nitrate, NO3-N 0.2 (SAFE)
Sulfate, SO4-S 3
Chloride, Cl 9
Carbonate, CO3 < 1.0
Bicarbonate, HCO3 244
Total Alkalinity, CaCO3 201
Total Phosphorus, P 0.02
Total Iron, Fe < 0.01