MPBeer
Well-Known Member
- Joined
- Dec 12, 2017
- Messages
- 98
- Reaction score
- 10
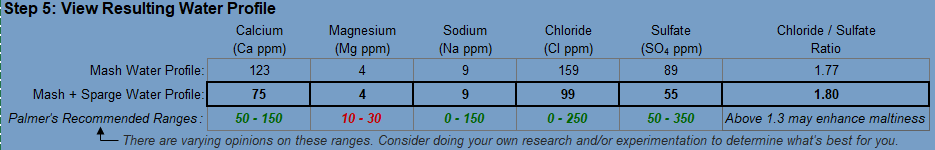
I'm using EZ water calculator, and I'm always confused if I should look at the Mash Water Profile, or Mash + Sparge Water Profile. I'm trying to brew a NEIPA and I'm looking for 100~150 Cl : 50 SO4. I added 2 gram of Gypsum and 4 gram of CaCl2, and I've already got tons of Chloride and Sulfate at my Mash Water Profile. However, after the sparge water is added, the number goes down significantly. Should I adjust my addition looking at the latter one and add more?
















![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)






















