- Joined
- Dec 19, 2013
- Messages
- 322
- Reaction score
- 398
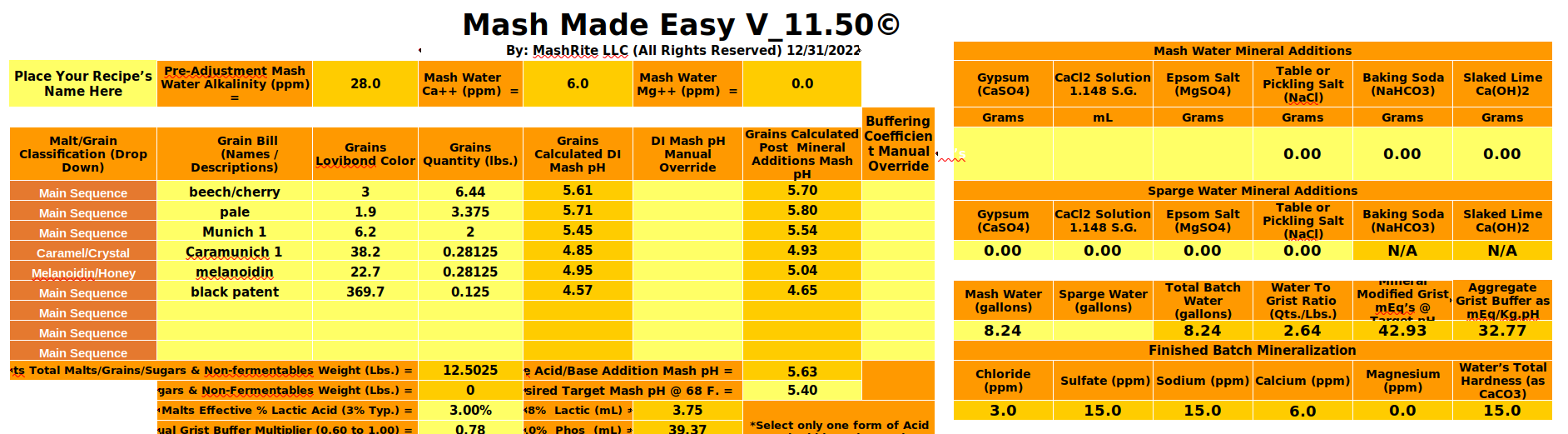
I am planning to brew @passedpawn Rauchbier linked below. When I build the recipe in BrewFather it lists my PH as 4.96 before any adjustments. This is using my tap water which is usually very good for brewing and is always higher ph before water treatment. Is this the smoked malt causing it? I set the water profile as Marzen thinking that'd be a good base. Problem is I can't get the PH above 5 without raising the sodium or calcium too high. Also the Bicarbonate is high no matter what I do. I've got Gypsum, Calcium Chloride, Epsom Salt, Canning Salt, Slaked Lime, and Baking Soda. Everyone says avoid Chalk so now I don't really know what to do. Do I leave it at 5? Do I push the Sodium and/or Calcium higher? Do I try a different mineral? Should I use a different base profile?
https://www.homebrewtalk.com/threads/cherry-beech-smokebeer-many-awards.157818/
Tap
Ca=6 Mg=0 Na=15 Cl=3 SO=15 HCO=34
Target
Ca=62 Mg=15 Na=50 Cl=100 SO=50 HCO=80
Best I can get at ph of 5
Ca=68 Mg=5 Na=50 Cl=101 SO=52 HCO=125
https://www.homebrewtalk.com/threads/cherry-beech-smokebeer-many-awards.157818/
Tap
Ca=6 Mg=0 Na=15 Cl=3 SO=15 HCO=34
Target
Ca=62 Mg=15 Na=50 Cl=100 SO=50 HCO=80
Best I can get at ph of 5
Ca=68 Mg=5 Na=50 Cl=101 SO=52 HCO=125
























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)