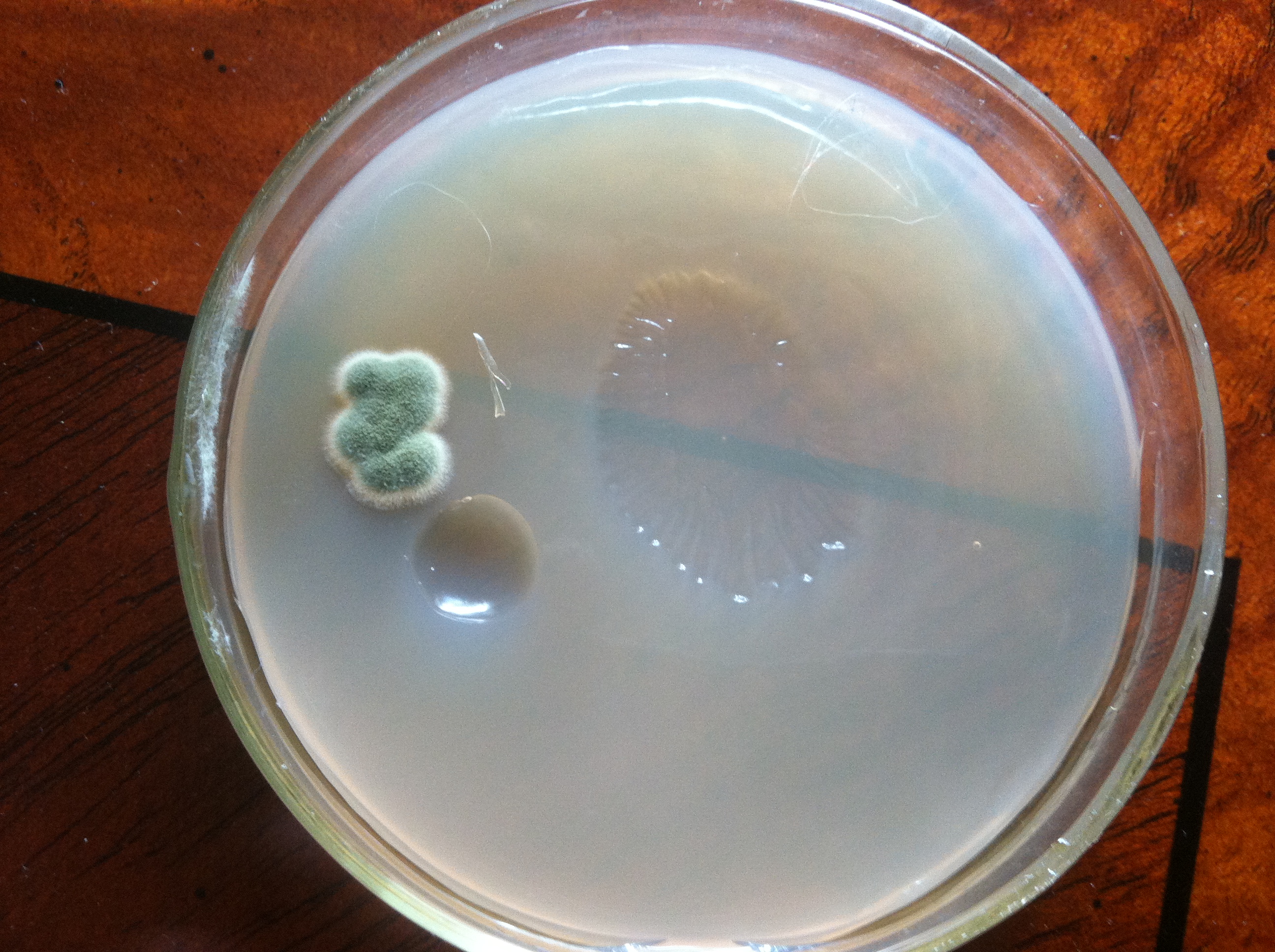
Very interesting, I can picture this creating the streaked film on top of the beer. It's too bad it takes so long of video to show the growth. Great effort PassedPawn.
This new software I'll be using this weekend will make the time lapse stuff much easier (no more video editing). It takes single snapshots at intervals, exactly what I need; i.e., one snapshot per minute over a day. Unfortunately it's going to cost me $35 (I tried some free utilities but they were not very impressive).
One problem I have is that during growth I have to occasionally adjust focus. I trust the X/Y stage of my scope, so I guess there is vertical movement of the media (agar substrate) somehow. The living culture might just be rising too, not sure yet. I had to adjust the focus a couple of times during that video and it created some weirdness in the final time lapse video.
























































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)