So close one eye......I have to do that after drinking sometimes.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Pics of Yeast under my new microscope
- Thread starter passedpawn
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
wegz15
Well-Known Member
I just wanted to know if the scope was messed up or my eyes.
I always do that when I get drunk too.
I always do that when I get drunk too.
PoppinCaps
Well-Known Member
Try adjusting the width between the eye pieces while looking thru it. Should merge the 2 drunken images. 
wegz15
Well-Known Member
I tried that. It got too wide (pause for that's what she said comment)
my other scope doesn't do double vision but it is only a 200x.
I did receive my hemocytometer in the mail the other day too. I need to clean the scope, its been in the garage for at least 4 years.
my other scope doesn't do double vision but it is only a 200x.
I did receive my hemocytometer in the mail the other day too. I need to clean the scope, its been in the garage for at least 4 years.
What type of stain are you looking to get? I count yeast a lot for viability and I am in the process of switching from methylene blue to alkaline methylene violet. The dye used to make the latter is methylene violet 3RAX. I have only really found it a Sigma Aldrich and they require you be a company or something order. I also believe that the dye is mixed into a glycine buffered alkaline solution. I made a little order mix up last time I sent something to the purchasing department, and neglected to include the dry dye in my order list. The cool thing is with the 1gram of dry dye you get a TON of solution to use. You simply take .1gram into 1L and 100ml of that stock is used to make 1L of actual die. So as you can see for $25 1gram of dye goes a real long ******* way when you're using 1ml per sample. Your other option would be to order 100ml worth I think from White Labs. It's expensive compared to buying the ingredients in terms of what you get but it'd sure be easier.
My school has a very high end scope that I just found out has a sweet ass camera attached. As soon as this semester is over (its finals week now) my prof will show me how to not screw it up since it's like $50k, and then they'll set me free to take pictures of whatever I want.
If you need any help or info let me know.
I found the dry dye here. 1g makes 1000 ml
But because of the small amount I need, the White Labs solution is probably the way I'll go. $12 for 50ml.
I think that while I'm waiting for that to come in the mail, I'll pick up some Methylene Blue (aquarium store).
You sure it makes 1000ml and not 1000L?
http://www.whitelabs.com/beer/Alkaline_Methylene_Violet_Solution.pdf
http://www.whitelabs.com/beer/Alkaline_Methylene_Violet_Solution.pdf

$719.00
$799.00
EdgeStar KC2000TWIN Full Size Dual Tap Kegerator & Draft Beer Dispenser - Black
Amazon.com

$20.94
$29.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.com

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
Guangshui Weilu You Trading Co., Ltd

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$479.00
$559.00
EdgeStar KC1000SS Craft Brew Kegerator for 1/6 Barrel and Cornelius Kegs
Amazon.com
You sure it makes 1000ml and not 1000L?
http://www.whitelabs.com/beer/Alkaline_Methylene_Violet_Solution.pdf
0.1g makes 100ml. So, 1g makes 1000ml, right?
Right 1g makes 1L of stock. Each 10ml of stock makes 100ml of dye. Sorry each 1g makes 10L of dye not 1000L. The stuff you're buying at White Labs is the diluted stock. Not the .1g diluted solution.
Right 1g makes 1L of stock. Each 10ml of stock makes 100ml of dye. Sorry each 1g makes 10L of dye not 1000L. The stuff you're buying at White Labs is the diluted stock. Not the .1g diluted solution.
Thanks. I was unsure of the second dilution. I'm not a chemist, and I have very limited ability to do anything related to chemistry
You'll have to let me know how it works because I don't have dye to use yet. I have just under 100ml of methylene blue to use yet.
mewithstewpid
Well-Known Member
You need a dye like trypan blue to label dead cells. Invitrogen sells this aka gibco, for like 20$ for 100ml I believe
Edit- never mind this has been discussed
Edit- never mind this has been discussed
I am looking for a microscope also and was thinking of using one that hooks up via usb. Does anyone think this type of microscope would allow for enough resolution to see yeast buds or even just viability counting?
http://www.amazon.com/dp/B005GJBDM4/?tag=skimlinks_replacement-20
Is oil immersion really a big bonus to have? Thanks for any insight!
http://www.amazon.com/dp/B005GJBDM4/?tag=skimlinks_replacement-20
Is oil immersion really a big bonus to have? Thanks for any insight!
Last edited by a moderator:
I am looking at microscopes for this and wanted to see if anyone thinks this type of microscope would be sufficient to see yeast buds or even do cell counts for viability. It is a USB 40x to 800x but not oil immersion. I'm curious as to what kidn of quality I could expect.
http://www.amazon.com/dp/B005GJBDM4/?tag=skimlinks_replacement-20
Thanks for any insight!
http://www.amazon.com/dp/B005GJBDM4/?tag=skimlinks_replacement-20
Thanks for any insight!
Last edited by a moderator:
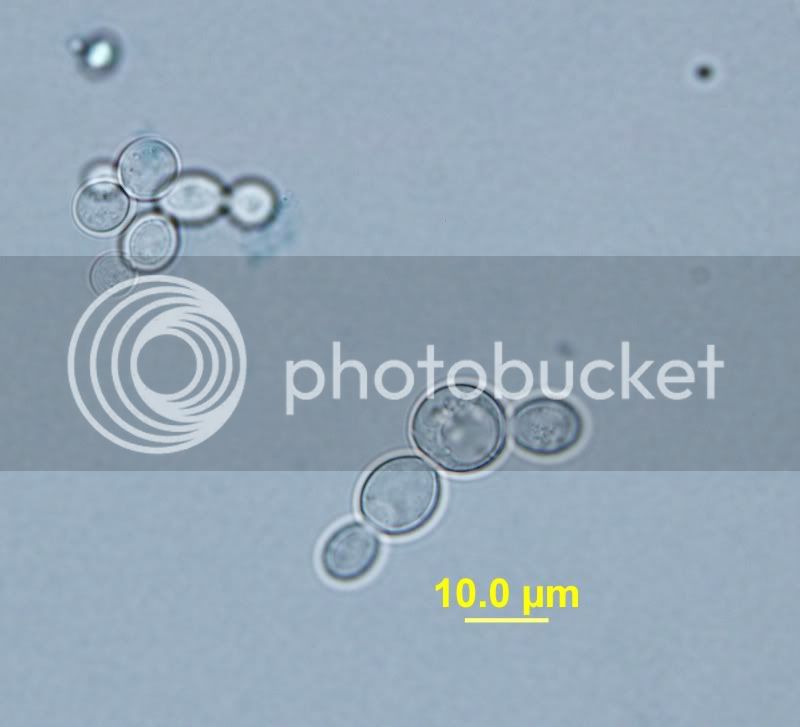
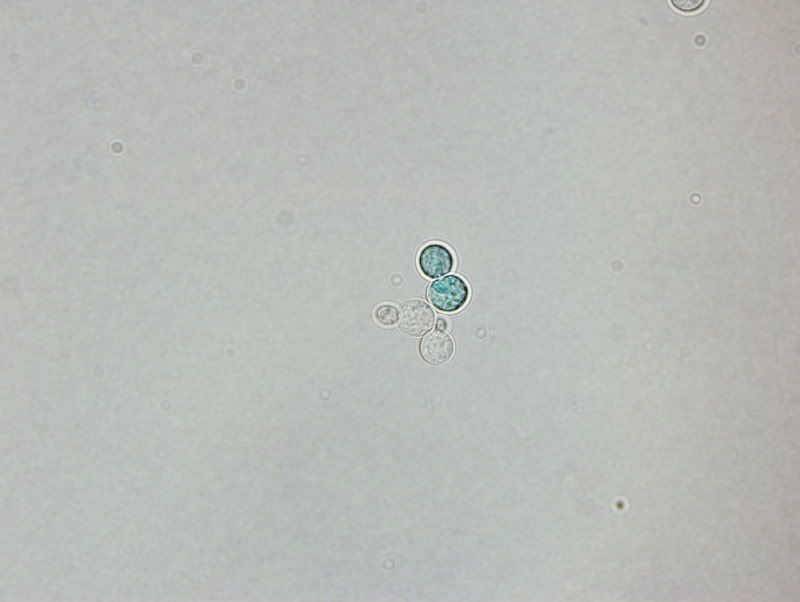
I finally got on my school's scope with the camera. You can see in the one with the dye the budding yeast are more concerned about budding and will not expel/metabolize the dye. They are alive and well they're just not concerned about anything but budding at that point.
Those are great pics! I wish mine was trinocular.
They should be great, that damn scope is like $30,000. I want to see if I can take some florescent pictures. I just need to get a florescent dye that will bind to certain proteins on the cell surface or interior. This scope has a florescence attachment that can take some really cool pictures.
They should be great, that damn scope is like $30,000. I want to see if I can take some florescent pictures. I just need to get a florescent dye that will bind to certain proteins on the cell surface or interior. This scope has a florescence attachment that can take some really cool pictures.
can you do phase contrast?
I do believe. I just need to figure it out. I'd like to take some pics in phase contrast of brett and other yeast.
I am looking at microscopes for this and wanted to see if anyone thinks this type of microscope would be sufficient to see yeast buds or even do cell counts for viability. It is a USB 40x to 800x but not oil immersion. I'm curious as to what kidn of quality I could expect.
Amazon.com: 800x 2mp 8-led Usb Digital Microscope with Handsfree Stand (Support Windows 7(support 32 Bit Only!)/vista/xp/2000/mac Os X 10.5 or Above): Camera & Photo
Thanks for any insight!
You don't need oil immersion. That is usually only necessary for objective magnifications of 100x or greater. With a 40x objective (and 10x eyepiece), you have 400x which is terrific for counting on a hemocytometer.
With an oil immersion objective, you might really get a good closup look at yeast budding, but you'd see it without also. Cell morphology would be easier with oil immersion also.
That scope isn't going to do it for you. While the magnification is there, you need the type that is lighted from beneath (lamp -> condenser). Might want to read the reviews of anything you buy first.
Last edited by a moderator:
nebben
Well-Known Member
I've always been curious, do yeast by chance have an endoplasmic reticulum, or a golgi apparatus?
Just thinking back to some of my favorite terms from my biology days...
Just thinking back to some of my favorite terms from my biology days...
Yes.
I got my microscope a couple days ago and have been geeking out and having a ball doing it. Very cool to see my yeasties up close.
Can anyone suggest a good reference online or book for identifying different types of yeast cultures from each other and from bacteria? I have the Yeast book from Jamil and White but would like something a little more in depth. Thanks for any suggestions!
I took these pics of wild Reinart ale and a wild Avery beer with my phone camera lens held to the eye piece. It's a work in progress
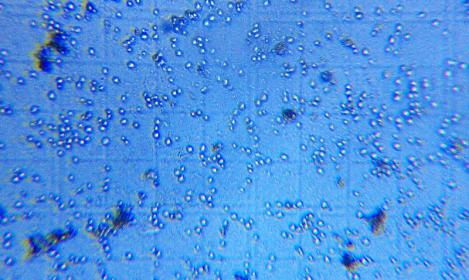
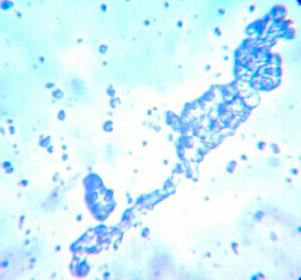
Can anyone suggest a good reference online or book for identifying different types of yeast cultures from each other and from bacteria? I have the Yeast book from Jamil and White but would like something a little more in depth. Thanks for any suggestions!
I took these pics of wild Reinart ale and a wild Avery beer with my phone camera lens held to the eye piece. It's a work in progress


PoppinCaps
Well-Known Member
Bacteria is easy, you can really only see them at 400x, they're super tiny compared to the yeast and dancing around. Hard to distinguish from small particles with Brownian motion, but it's obvious when there's an infection. Identifying different yeast is a tough one if you're only talking Saccharomyces, as they're roughly identical in looks, colony formation and color, etc. If you're talking wild yeast captured, some yeast will have hyphae (like mushroom "roots"), spores, crazy colors, etc. Then you could use an agar plate to distinguish soft white colonies from the crazy ones. Sorry, I don't have a good suggestion for reading material, but any fungal text is a good start.
Nice work with the camera phone!
Nice work with the camera phone!
Damn robh you took those pics with your phone? That's incredible.
I have the same desire as you, identifying yeast and bacteria. There's some good blogs out there with info. I'm on the road now (San Francisco) I'll post links when I get back.
I have the same desire as you, identifying yeast and bacteria. There's some good blogs out there with info. I'm on the road now (San Francisco) I'll post links when I get back.
Thanks for the info PoppinCaps. I'll check and see if my local library has any good fungal texts.
Passedpawn - I was pleasantly surprised at the quality too! I definitely have room for improvement, but I'm happy with that clarity considering how easy it was to do. I have an Android Evo with an 8 megapixel camera. The autofocus helps a lot. Please do send any links or references that you think might be helpful. The info you and others have provided on this forum have been a great help already! Thanks.
Passedpawn - I was pleasantly surprised at the quality too! I definitely have room for improvement, but I'm happy with that clarity considering how easy it was to do. I have an Android Evo with an 8 megapixel camera. The autofocus helps a lot. Please do send any links or references that you think might be helpful. The info you and others have provided on this forum have been a great help already! Thanks.
Here's a couple new pics I snapped today at school. They looked much better on the comp that snaps the photos but I guess the data transfer or the program I'm using to modify and crop do something to the quality. Also I'd like to note I don't know how accurate the scale is, but it can be used for size reference. I took all these pictures at the same magnification.
One pic is a wild yeast colony I pulled off a Lins Cupric Sulfate Medium. I think there where 3 total decent sized colonies. One separate and two colonies that merged together to equal about the size of the single. I have images of WLP510 that I am in the process of growing up from frozen stock as of yesterday.
Here's the yeast that grew on the LCSM.

Here's WLP510 for reference. You can see a nice tiny bud in the second picture.
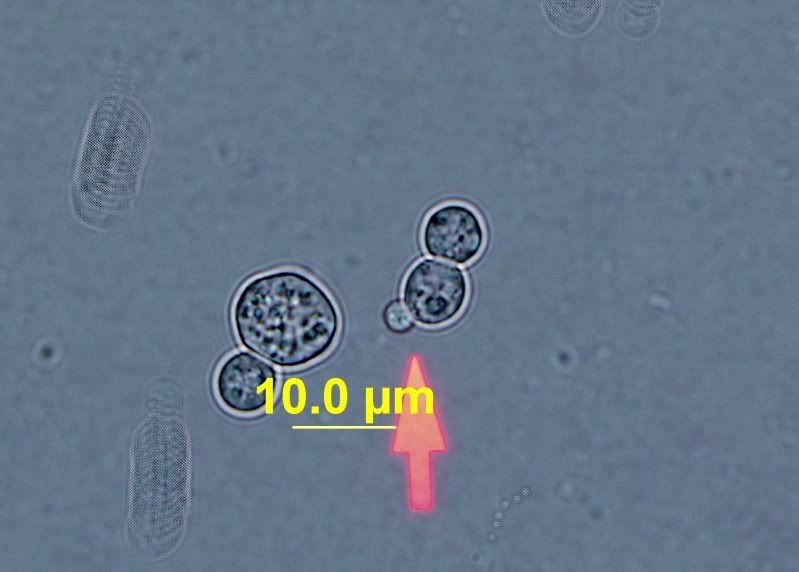

Then here's some of what I suspect to be brett. It was isolated and grown from a unknown culture on WLD medium.

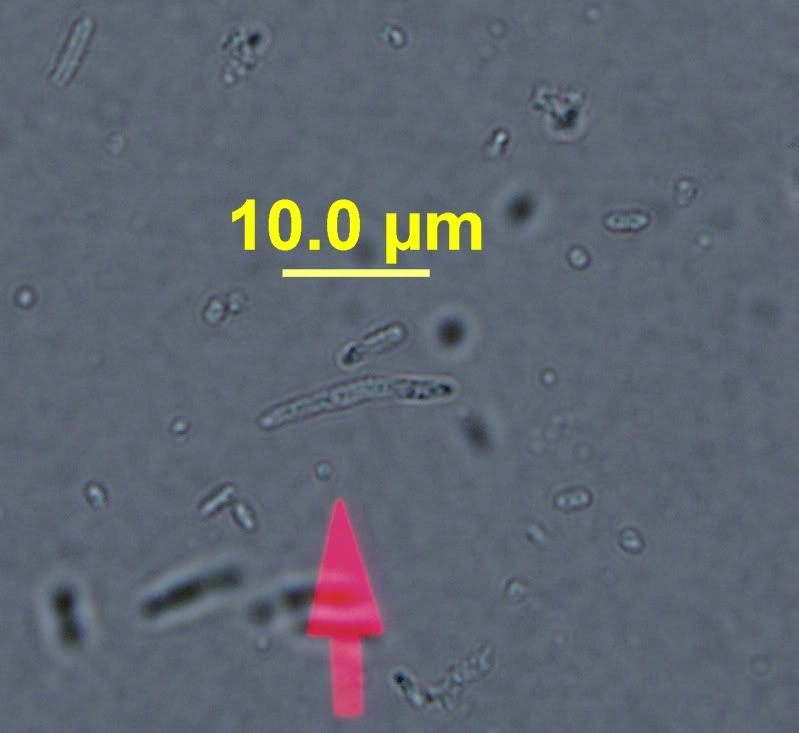

One pic is a wild yeast colony I pulled off a Lins Cupric Sulfate Medium. I think there where 3 total decent sized colonies. One separate and two colonies that merged together to equal about the size of the single. I have images of WLP510 that I am in the process of growing up from frozen stock as of yesterday.
Here's the yeast that grew on the LCSM.

Here's WLP510 for reference. You can see a nice tiny bud in the second picture.


Then here's some of what I suspect to be brett. It was isolated and grown from a unknown culture on WLD medium.



dstar26t
If it's worth doing, it's worth overdoing
Those pics are awesome. I'm finding that a microscope is a very important part of my brewery, not just another toy to play with.
I'm brewing a big Stout on Saturday and made a starter for it this past Tuesday. Looking for 765 Billion cells so I put 3 vials (WLP001) in a 4L starter. It was finished this morning so I did a cell count. Only 394 Billion cells! My microscope has officially saved it's first beer. I can easily supplement with some dry yeast now to get the correct pitching rate. Before the microscope, the only tool available to me was Jamil's calculator which it seems doesn't by itself insure the correct cell count.
I'm brewing a big Stout on Saturday and made a starter for it this past Tuesday. Looking for 765 Billion cells so I put 3 vials (WLP001) in a 4L starter. It was finished this morning so I did a cell count. Only 394 Billion cells! My microscope has officially saved it's first beer. I can easily supplement with some dry yeast now to get the correct pitching rate. Before the microscope, the only tool available to me was Jamil's calculator which it seems doesn't by itself insure the correct cell count.
Those pics are awesome. I'm finding that a microscope is a very important part of my brewery, not just another toy to play with.
I'm brewing a big Stout on Saturday and made a starter for it this past Tuesday. Looking for 765 Billion cells so I put 3 vials (WLP001) in a 4L starter. It was finished this morning so I did a cell count. Only 394 Billion cells! My microscope has officially saved it's first beer. I can easily supplement with some dry yeast now to get the correct pitching rate. Before the microscope, the only tool available to me was Jamil's calculator which it seems doesn't by itself insure the correct cell count.
How old were the vials? There's around 100B cells in a vial, so either your's weren't very viable or you had very little growth. How much DME did you add to the 4L?
I use mine on every starter. 1 drop of starter and a couple of minutes of counting.
I've played some more with video, but I haven't captured budding yeast yet. Once I do that, I'll time-lapse it and post here.
I've played some more with video, but I haven't captured budding yeast yet. Once I do that, I'll time-lapse it and post here.
dstar26t
If it's worth doing, it's worth overdoing
Vials dated 7/11/12 and kept cold at homebrew shop. I drove the vials home from the shop in a cooler with ice. Let them warm to room temp before pitching. 3810 grams water and 381 grams DME on stir plate for 36 hours at 66ºF. Treated the water with K2S2O5 at the recommended rate (.44g/20gal) and used the recommended amount of Wyeast Yeast Nutrient (2.2g/5gal).
I'm at a loss as to why they didn't multiply more, glad I checked though!
I'm at a loss as to why they didn't multiply more, glad I checked though!
Similar threads
- Replies
- 2
- Views
- 424
- Replies
- 2
- Views
- 961
Latest posts
-
-
-
Spike Conical Heater fails again--second in 3 years--ideas?
- Latest: Indian_villager
-
-
-



































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)










