- Joined
- Jun 2, 2008
- Messages
- 64,955
- Reaction score
- 16,524
I do appreciate that he has an enthusiasm for beer and obviously puts a lot of time and effort into his website. That aspect is to be commended.
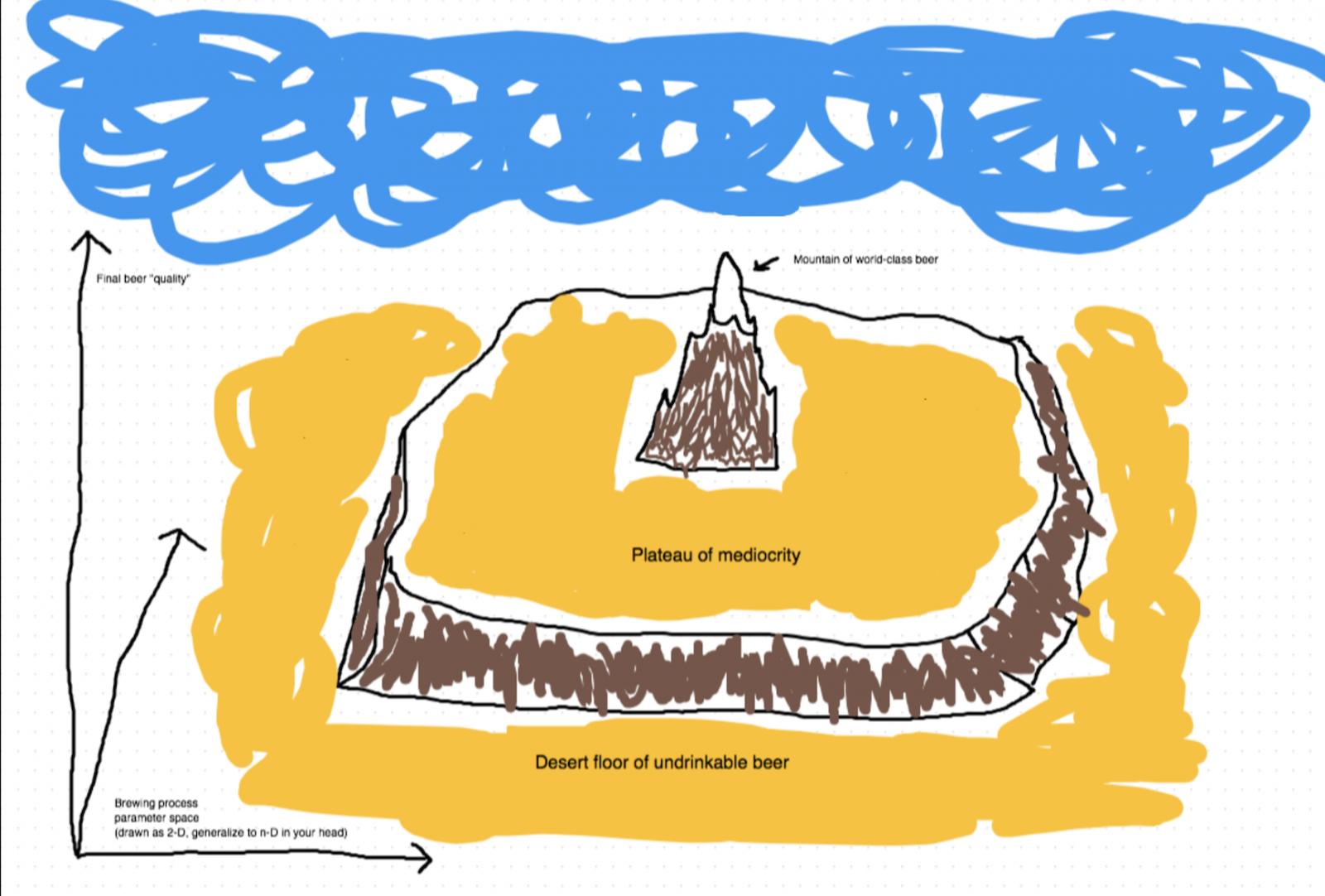
However, I do think he portrays his "experiments" in a quasi-scientific way that may lead some readers, especially those without a scientific background, to actually put stock in what he says. Most of his experiments suggest that many well established bad brewing practices don't have negative consequences. That does a tremendous disservice to the home brewing community.
For example?



![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)