Nordic Brewer
Member
- Joined
- Feb 21, 2018
- Messages
- 8
- Reaction score
- 0
Ph Change (drift) during mashing.
In different thread discussions, pH-drift has been explained as:
- pH meter is drifting or pH meter instability
- It takes time to reach chemical equilibrium in the mash, measurement taken to early.
- Or there may be some mash chemistry that is not taken into account in the calculators and spreadsheet used by many brewers.
I have not given much though to this issue until I did some water-adjustment and pH readings myself and experienced the same phenomenon.
Here is the result from my last brewday.
Later I found a scientific paper describing the same phenomenon. A discussion around that is found after my description of my pH readings.
Preconditions:
1. NO SPARGE, total water volume 30.2 litre
2. Temperature step infusion mashing with Speidel Braumeister equipment (electrical heating together with in-build water pump and temp regulator).
3. Source Water profile:
- Ca++ 6.25 mg/l
- Mg++ 1.50 mg/
- Na+ 0 mg/l
- Cl- 2.46 mg/l
- SO4-- 4.34 mg/l
- Alkalinity as CaCO3 12.5 ppm
- pH 7.18
- Ione balance 0.07 mEq/l
Water profile adjustment:
- + 3.4g CaCl2*2H20
- + 9.05g Lactic Acid 80%
Grist (Grist Crush 1.3mm):
- 4.6kg Pilsner malt
- 0.3kg Wheat malt
- 0.3kg Cara Pils
Using Brewer's friend water calculator gives an estimated mash pH = 5,20
4. Using Tesco pH meter with temp control with accuracy and precision +/- 0.01 pH.
- Automatic reading when measurement is stable.
- Measurement is done when sample is cooled down to 25C.
- pH meter calibrated in 2 step buffer before mashing
- pH meter turned on for each measurement to avoid drifting.
- pH meter is controlled against calibration buffer after mashing is completed.
Step-Mashing and Ph readings:
Dough-in at 37C.
- 20 min at 40C => pH= 5.22
- 10 min at 55C => No pH reading
- 0 min at 66C => pH= 5.46
- 60 min at 66C => pH= 5.51
- 20 min at 72C => pH= 5.51
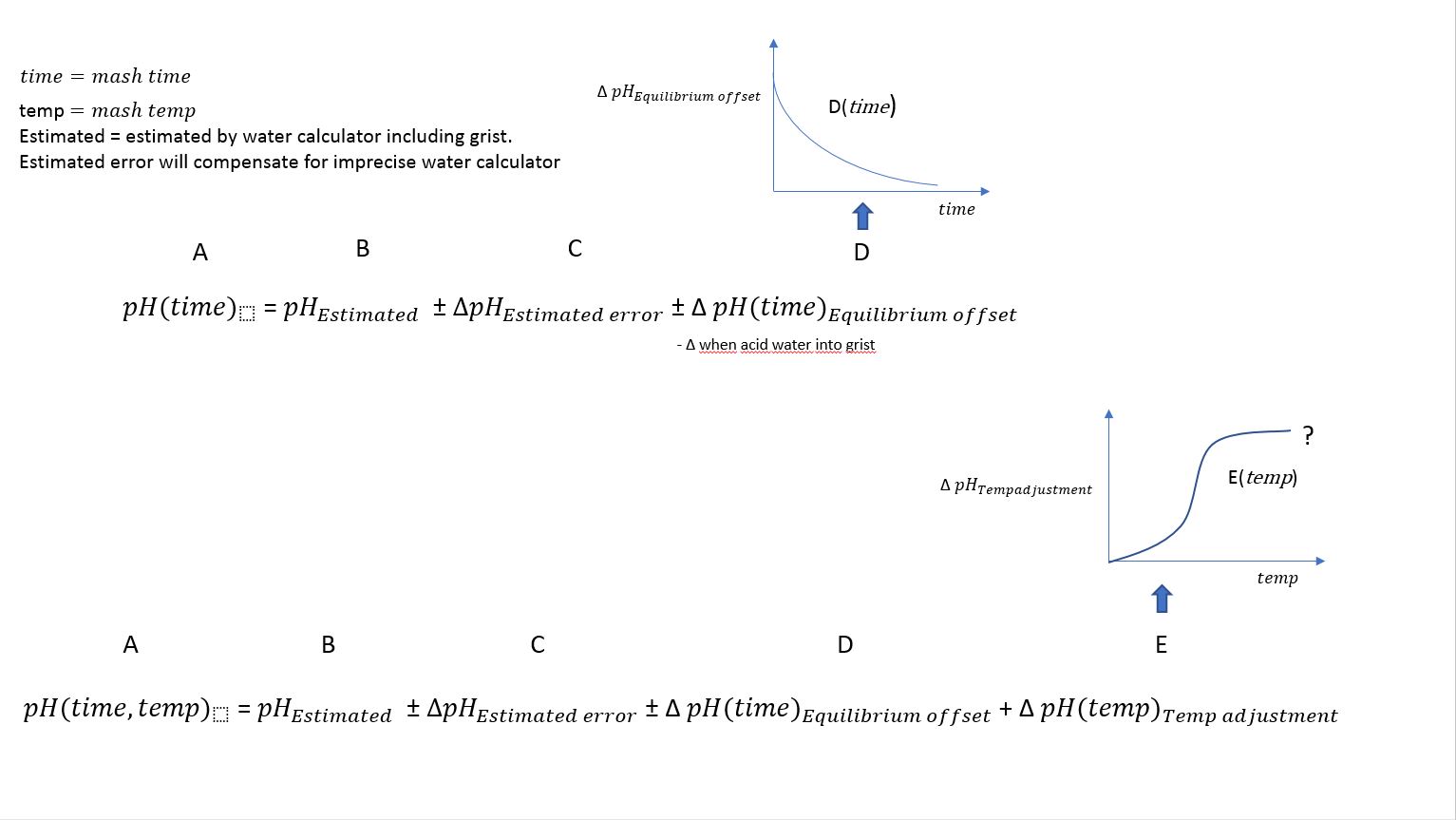
What we see is that the pH continue to drift upward during the mashing and stabilize at 5.51.
The interesting part is the correlation between actual Mash pH = 5.22 after 20 min rest and estimated pH.
Estimated pH in Brewer's friend calculator is 5.20.
In other words, estimated and empirical pH reading is within accepted values at 40C.
It also seems that empirical mash buffer capacity is measured via tritration and done at a given temperature.
ref article by A.J. deLange 2013: http://wetnewf.org/pdfs/estimating-mash-ph.html
I believe that results from these and similar kind of empirical measurement again is used in different calculators and spreadsheets.
In different thread discussions, pH-drift has been explained as:
- pH meter is drifting or instability
- It takes time to reach chemical equilibrium in the mash.
- Or there may be some mash chemistry that is not taken into account in the calculators and spreadsheet used by many brewers.
I have been searching for scientific papers, where pH drift in the mash has been observed.
I found this one:
Recipitation of Protein During Mashing: Evaluation of the Role of Calcium, Phosphate, and Mash pH
by M. J. Lewis and N. Nelson Wahnon, Department of Food Science and Technology, University of California, Davis 95616
http://www.agraria.com.br/extranet/...precipitacao_de_proteina_na_mostura_-_ing.pdf
The interesting thing about this article is that it seems to correlate with my observed pH-shift measurement example above.
The experiment in the article was doing step mashing (also single step) and what they found out was this:
- pH was creeping upward with time and maxing out at 70C before creeping down again
- When they mixed CaCl at different concentrations into the mash the pH-creep function shifted down as expected (lower pH)
- They also observed a sharp increase in the concentration of dissolved proteins from 40C and into the beginning of the saccharification temp interval
and then decreasing sharply after.
- Ca++ concentration had a decrease over time.
- They also did a so called "Grain out mash" in parallel, where all the grains and particles are filtered out of the mash and then do the mashing.
- For the "Grain out mash" they found that both Ca++ and pH did not drift over time but dissolved protein pattern remained the same.
What does that mean? It could mean that dissolved protein concentration does not have impact on observed pH drift.
The article does not conclude over the cause of the pH drift over time but points to solid material left behind in the mash when doing "grain out" mash.
So what could the cause of pH increase over time and temp increase be, if not caused by:
- pH meter is drifting or instability
- It takes time to reach chemical equilibrium in the mash.
We know there is a lot of particles and husk material in the mash together with dissolved and undissolved b-glucans and starch.
It is also known that beta-glucans and starch can be Acid Hydrolyzed but to which degree this take place in the mash is unknown.
In hydrolysis a larger molecule is broken down into simpler substances by the addition of water molecules.
When this process is carried out in the presence of acid, it is called Acid hydrolysis.
These reactions also speed up with increasing temp.
It is also known in organic chemistry that many acids can function as sources for the protons in this type of reactions such as phosphoric acid.
If this is the case here, free protons H+/H30+ could go into the acid hydrolysis reaction and we would observe that Ph decrease over time with increasing temp.
If acid hydrolysis is increasing with temp then the Ca++/phosphate acid equilibrium will also be influenced, which again will have impact on the Ca++ consentration:
A sharp decrease of Ca++ consentration was also observed with increasing mash temp in the study referred above.
Well what is the practical impact of this if the hypotesis is valid?
- Measure pH after ca 20min mashing before comparing with your estimated/calculated values and eventually do adjustments.
- Depending on mash schedule and method: The timing of acid addition is something to consider.
Looking forward to get some feedback on this.
In different thread discussions, pH-drift has been explained as:
- pH meter is drifting or pH meter instability
- It takes time to reach chemical equilibrium in the mash, measurement taken to early.
- Or there may be some mash chemistry that is not taken into account in the calculators and spreadsheet used by many brewers.
I have not given much though to this issue until I did some water-adjustment and pH readings myself and experienced the same phenomenon.
Here is the result from my last brewday.
Later I found a scientific paper describing the same phenomenon. A discussion around that is found after my description of my pH readings.
Preconditions:
1. NO SPARGE, total water volume 30.2 litre
2. Temperature step infusion mashing with Speidel Braumeister equipment (electrical heating together with in-build water pump and temp regulator).
3. Source Water profile:
- Ca++ 6.25 mg/l
- Mg++ 1.50 mg/
- Na+ 0 mg/l
- Cl- 2.46 mg/l
- SO4-- 4.34 mg/l
- Alkalinity as CaCO3 12.5 ppm
- pH 7.18
- Ione balance 0.07 mEq/l
Water profile adjustment:
- + 3.4g CaCl2*2H20
- + 9.05g Lactic Acid 80%
Grist (Grist Crush 1.3mm):
- 4.6kg Pilsner malt
- 0.3kg Wheat malt
- 0.3kg Cara Pils
Using Brewer's friend water calculator gives an estimated mash pH = 5,20
4. Using Tesco pH meter with temp control with accuracy and precision +/- 0.01 pH.
- Automatic reading when measurement is stable.
- Measurement is done when sample is cooled down to 25C.
- pH meter calibrated in 2 step buffer before mashing
- pH meter turned on for each measurement to avoid drifting.
- pH meter is controlled against calibration buffer after mashing is completed.
Step-Mashing and Ph readings:
Dough-in at 37C.
- 20 min at 40C => pH= 5.22
- 10 min at 55C => No pH reading
- 0 min at 66C => pH= 5.46
- 60 min at 66C => pH= 5.51
- 20 min at 72C => pH= 5.51
What we see is that the pH continue to drift upward during the mashing and stabilize at 5.51.
The interesting part is the correlation between actual Mash pH = 5.22 after 20 min rest and estimated pH.
Estimated pH in Brewer's friend calculator is 5.20.
In other words, estimated and empirical pH reading is within accepted values at 40C.
It also seems that empirical mash buffer capacity is measured via tritration and done at a given temperature.
ref article by A.J. deLange 2013: http://wetnewf.org/pdfs/estimating-mash-ph.html
I believe that results from these and similar kind of empirical measurement again is used in different calculators and spreadsheets.
In different thread discussions, pH-drift has been explained as:
- pH meter is drifting or instability
- It takes time to reach chemical equilibrium in the mash.
- Or there may be some mash chemistry that is not taken into account in the calculators and spreadsheet used by many brewers.
I have been searching for scientific papers, where pH drift in the mash has been observed.
I found this one:
Recipitation of Protein During Mashing: Evaluation of the Role of Calcium, Phosphate, and Mash pH
by M. J. Lewis and N. Nelson Wahnon, Department of Food Science and Technology, University of California, Davis 95616
http://www.agraria.com.br/extranet/...precipitacao_de_proteina_na_mostura_-_ing.pdf
The interesting thing about this article is that it seems to correlate with my observed pH-shift measurement example above.
The experiment in the article was doing step mashing (also single step) and what they found out was this:
- pH was creeping upward with time and maxing out at 70C before creeping down again
- When they mixed CaCl at different concentrations into the mash the pH-creep function shifted down as expected (lower pH)
- They also observed a sharp increase in the concentration of dissolved proteins from 40C and into the beginning of the saccharification temp interval
and then decreasing sharply after.
- Ca++ concentration had a decrease over time.
- They also did a so called "Grain out mash" in parallel, where all the grains and particles are filtered out of the mash and then do the mashing.
- For the "Grain out mash" they found that both Ca++ and pH did not drift over time but dissolved protein pattern remained the same.
What does that mean? It could mean that dissolved protein concentration does not have impact on observed pH drift.
The article does not conclude over the cause of the pH drift over time but points to solid material left behind in the mash when doing "grain out" mash.
So what could the cause of pH increase over time and temp increase be, if not caused by:
- pH meter is drifting or instability
- It takes time to reach chemical equilibrium in the mash.
We know there is a lot of particles and husk material in the mash together with dissolved and undissolved b-glucans and starch.
It is also known that beta-glucans and starch can be Acid Hydrolyzed but to which degree this take place in the mash is unknown.
In hydrolysis a larger molecule is broken down into simpler substances by the addition of water molecules.
When this process is carried out in the presence of acid, it is called Acid hydrolysis.
These reactions also speed up with increasing temp.
It is also known in organic chemistry that many acids can function as sources for the protons in this type of reactions such as phosphoric acid.
If this is the case here, free protons H+/H30+ could go into the acid hydrolysis reaction and we would observe that Ph decrease over time with increasing temp.
If acid hydrolysis is increasing with temp then the Ca++/phosphate acid equilibrium will also be influenced, which again will have impact on the Ca++ consentration:
A sharp decrease of Ca++ consentration was also observed with increasing mash temp in the study referred above.
Well what is the practical impact of this if the hypotesis is valid?
- Measure pH after ca 20min mashing before comparing with your estimated/calculated values and eventually do adjustments.
- Depending on mash schedule and method: The timing of acid addition is something to consider.
Looking forward to get some feedback on this.








































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
















