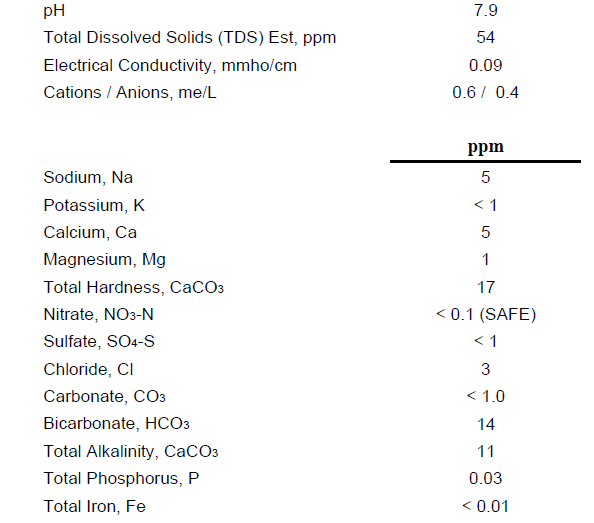
I've been brewing all grain for quite a while but other than PH buffers, have never attempted to make a lot of adjustments to the brewing water. I'm just completing the build of my new EHERMS brewery and wish to step up my game a bit. Also, I'm brewing with a different water source than in the past and hope to pick the brains of some of the amazing people here on what to expect from it. I have had my water tested by ward labs and have very soft water. My concern is that it may be too close to RO water and may need other mineral/salt additions. I'm planning on following ajdelange's "Brewing water chemistry primer" guidelines for adjusting the mash, but was interested in getting some opinions on weather or not I should be making any other adjustments to either the sparge water or the boil, and if so, what additions you would suggest. I have purchased a PH meter and plan to monitor the PH throughout the brew. I'm planning a fairly basic pale ale for the first brew, so there won't be any roasted grains. Here are the results of the testing, any opinions would be greatly appreciated!!!