Hi all
I have brewed several recipes but I never looked at the water analysis. I want to step up my game and modify the water parameters for the first time to improve the recipes and brew results. However, I have used several calculators (Brewersfriends, EZwatercalculator, Brun'water) but each time they give me different results.
Here's my recipe, it's meant as an easy to drink brown ale with notes of caramel, toffee and a hint of chocolate. I chose Willammette and Saaz for earthy/fruity hints.
Grainfather G30 - Micro
80% efficiency
Batch Volume: 12 L (3.17 gallons)
Boil Time: 60 min
Mash Water: 11.87 L (3.13 gallons)
Sparge Water: 7.15 L (1.88 gallons)
Total Water: 19.02 L (5.02 gallons)
Boil Volume: 16.54 L (4.36 gallons)
Pre-Boil Gravity: 1.052
Vitals
Original Gravity: 1.066
Final Gravity: 1.012
IBU (Tinseth): 21
BU/GU: 0.32
Color: 36 EBC
Mash
Temperature — 65 °C (149 °F) — 60 min
Mash Out — 75 °C (167 °F) — 10 min
Malts (3.099 kg)
2 kg (61.6%) — The Swaen Swaen Vienna — Grain — 10 EBC
1000 g (30.8%) — Weyermann Pilsner — Grain — 3.3 EBC
66 g (2%) — Weyermann Carafa Special II — Grain — 1100 EBC
34 g (1%) — Dingemans Special B — Grain — 290 EBC
Other (150 g)
150 g (4.6%) — Candi Syrup Candi Syrup, D-45 — Sugar — 88.5 EBC
Hops (25 g)
16 g (17 IBU) — Willamette 5.5% — Boil — 60 min
6 g (3 IBU) — Willamette 5.5% — Boil — 15 min
3 g (1 IBU) — Saaz 4.5% — Boil — 15 min
Miscs
2 g — Irish Moss — Boil — 10 min
Yeast
1 pkg — White Labs WLP530 Abbey Ale 80%
Fermentation
Primary — 20 °C — 7 days
Secondary — 20 °C — 10 days
Cold Crash — 5 °C — 2 days
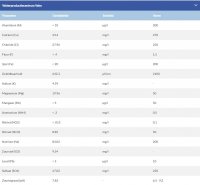
Here's my tap water results:
Calcium: 43
Magnesium: 20
Sodium: 83
Chloride: 28
Sulfate: 47
CaCO3: 160
Bicarbonates: 195
pH: 7,8
I was thinking of diluting this with distilled water by 50% and then adding 1.6g CaSO4, 3.2g CaCl2 and 1.6g MgSO4 and 1ml lactic acid of 80%. According to EZwatercalculator this shoud give me a pH of about 5,48 which is fine + the following parameters:
Calcium: 86
Magnesium: 18
Sodium: 41
Chloride: 95
Sulfate: 103
However, when I use Brewersfriends I need to use way more lactic acid to achieve this pH and when I use Bru'n I get the error that my pH will be lower then 5 when I'm not even using the lactic acid.
Am I doing something wrong or misinterpreting something? Any help is welcome.
I have brewed several recipes but I never looked at the water analysis. I want to step up my game and modify the water parameters for the first time to improve the recipes and brew results. However, I have used several calculators (Brewersfriends, EZwatercalculator, Brun'water) but each time they give me different results.
Here's my recipe, it's meant as an easy to drink brown ale with notes of caramel, toffee and a hint of chocolate. I chose Willammette and Saaz for earthy/fruity hints.
Grainfather G30 - Micro
80% efficiency
Batch Volume: 12 L (3.17 gallons)
Boil Time: 60 min
Mash Water: 11.87 L (3.13 gallons)
Sparge Water: 7.15 L (1.88 gallons)
Total Water: 19.02 L (5.02 gallons)
Boil Volume: 16.54 L (4.36 gallons)
Pre-Boil Gravity: 1.052
Vitals
Original Gravity: 1.066
Final Gravity: 1.012
IBU (Tinseth): 21
BU/GU: 0.32
Color: 36 EBC
Mash
Temperature — 65 °C (149 °F) — 60 min
Mash Out — 75 °C (167 °F) — 10 min
Malts (3.099 kg)
2 kg (61.6%) — The Swaen Swaen Vienna — Grain — 10 EBC
1000 g (30.8%) — Weyermann Pilsner — Grain — 3.3 EBC
66 g (2%) — Weyermann Carafa Special II — Grain — 1100 EBC
34 g (1%) — Dingemans Special B — Grain — 290 EBC
Other (150 g)
150 g (4.6%) — Candi Syrup Candi Syrup, D-45 — Sugar — 88.5 EBC
Hops (25 g)
16 g (17 IBU) — Willamette 5.5% — Boil — 60 min
6 g (3 IBU) — Willamette 5.5% — Boil — 15 min
3 g (1 IBU) — Saaz 4.5% — Boil — 15 min
Miscs
2 g — Irish Moss — Boil — 10 min
Yeast
1 pkg — White Labs WLP530 Abbey Ale 80%
Fermentation
Primary — 20 °C — 7 days
Secondary — 20 °C — 10 days
Cold Crash — 5 °C — 2 days
Here's my tap water results:
Calcium: 43
Magnesium: 20
Sodium: 83
Chloride: 28
Sulfate: 47
CaCO3: 160
Bicarbonates: 195
pH: 7,8
I was thinking of diluting this with distilled water by 50% and then adding 1.6g CaSO4, 3.2g CaCl2 and 1.6g MgSO4 and 1ml lactic acid of 80%. According to EZwatercalculator this shoud give me a pH of about 5,48 which is fine + the following parameters:
Calcium: 86
Magnesium: 18
Sodium: 41
Chloride: 95
Sulfate: 103
However, when I use Brewersfriends I need to use way more lactic acid to achieve this pH and when I use Bru'n I get the error that my pH will be lower then 5 when I'm not even using the lactic acid.
Am I doing something wrong or misinterpreting something? Any help is welcome.
Last edited:













































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)