You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
NaOH for TA test... a question for the chemists.
- Thread starter Chalkyt
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
bracconiere
Jolly Alcoholic - In Remembrance 2023
*
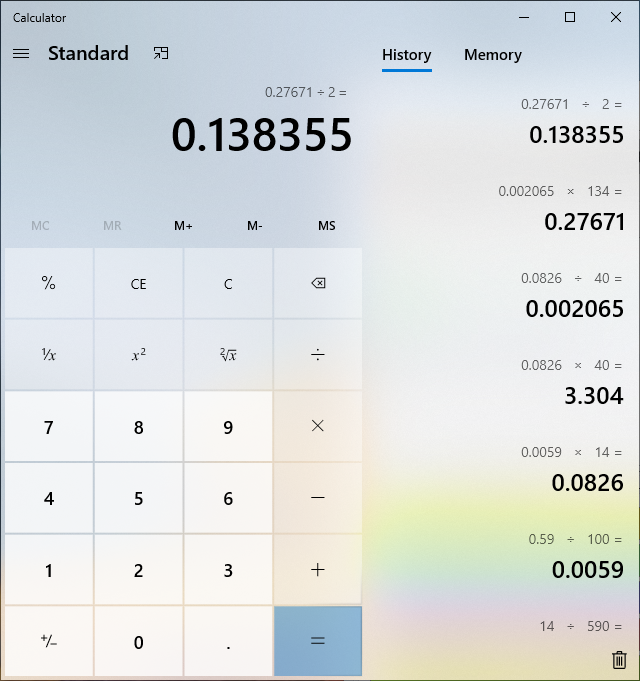
damn... i added 590mg's of lye to 100g's water...it took 14g's of the water to neutrilize 136 mg's malic acid...which would be 8mg's lye...which would be .002M times the mol weight of malic acid of 134g's... and it takes 2 mols of lye to neutrilize 1 mol of malic, so then i divided it by 2?
now i want someone else to tell me if i got this or not?

damn... i added 590mg's of lye to 100g's water...it took 14g's of the water to neutrilize 136 mg's malic acid...which would be 8mg's lye...which would be .002M times the mol weight of malic acid of 134g's... and it takes 2 mols of lye to neutrilize 1 mol of malic, so then i divided it by 2?
now i want someone else to tell me if i got this or not?
Attachments
Last edited:
bracconiere
Jolly Alcoholic - In Remembrance 2023
i think i got this down now... this was the same lye solution, added to water with a 'known' 33mg's of malic...it took 3g's of the lye water..and i'd say this is pretty damn acurrate then..
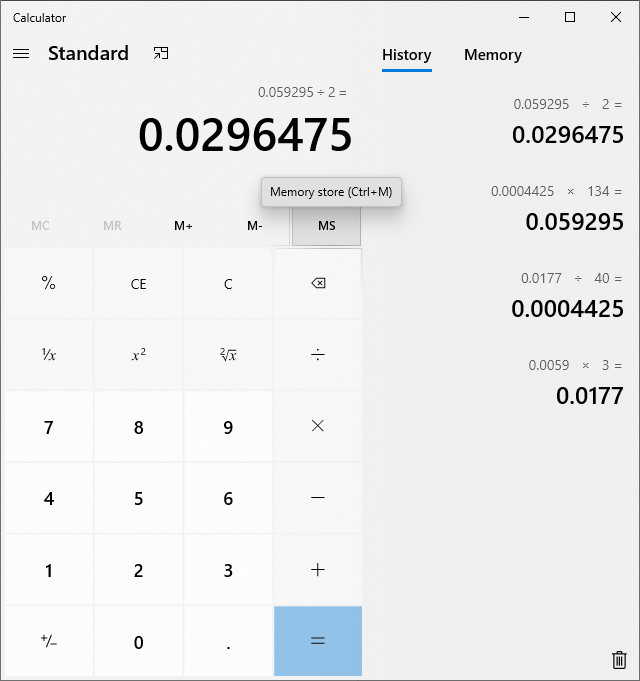
 there is the math clean version....
there is the math clean version....
edit: i should mention, i did this on a stir plate with a stir bar spinning while adding the lye solution....

 there is the math clean version....
there is the math clean version....edit: i should mention, i did this on a stir plate with a stir bar spinning while adding the lye solution....
Last edited:
bracconiere
Jolly Alcoholic - In Remembrance 2023
Ok i just figured out how to simplafie this for a cider maker....if you add 1g of lye to 200g's water, for every gram of the lye solution you add to your juice, there is 10mg's of malic acid....i just proved it on a known water malic acid solution i weighed out 20mg's of pure malic acid, and it took 2g's of the lye solution to turn the indicator pink.....(sorry 20mg's was hard enough to weigh out on my mg scale, couldn't prove it more presicly)
@Chalkyt Hope that makes it simple and easy!

@Chalkyt Hope that makes it simple and easy!

This point got lost in the discussion.
Making your own NaOH solution without standardizing it with KHP is an exercise in futility --- you will not get an accurate result because you cannot measure a known quantity of NaOH.
Potassium hydrogen phthalate (KHP) is not hygroscopic or volatile, so the amount can be accurately measured (unlike other acids being suggested above). If you can acquire some, I'll help you create a proper NaOH solution. Otherwise I wouldn't bother with testing TA using a bad NaOH solution.
This makes zero sense; one or both of your solutions are way off the mark.
There's a way to titrate your NaOH with citric acid IIRC, which is supposed to be easier to make accurate solutions of. It's described in detail in Dunn's Scientific Soapmaking.
https://www.amazon.com/Scientific-Soapmaking-Chemistry-Cold-Process/dp/1935652095
bracconiere
Jolly Alcoholic - In Remembrance 2023
There's a way to titrate your NaOH with citric acid IIRC, which is supposed to be easier to make accurate solutions of. It's described in detail in Dunn's Scientific Soapmaking.
https://www.amazon.com/Scientific-Soapmaking-Chemistry-Cold-Process/dp/1935652095
well i just whipped up that lye solution with non air tight stored lye, in ~200g's water lye it was actually 203-4g's...and the gram of lye was weighed on a milligram scale...i think it might have been a couple mg's shy of the full gram...
and it worked perfectly to neutrilize 20mg's malic acid....(kinda gave me a woody
 )
)i mean i'll do it again? but i think for pression, 1g in 200g's water is good enough....gives a 10mg perccsion point? if you saw me even trying to weigh out 20mg's malic acid on my milligram scale you would have gotten a good laugh....i don't think a few Mols of water at 18g's a mol is going to matter....

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$20.94
$29.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.com

$33.98
DYKWSWYX Heavy Duty Brewing Gloves (1 Pair) - 55CM Long Chemical Resistant Plastic Gloves for Beer & Wine Making, Cleaning, Homebrew Equipment Protection
wuhanshijiayangzhiyimaoyiyouxiangongsi

$76.92 ($2,179.04 / Ounce)
Brewing accessories 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304 Brewing accessories(Gas Hose Barb)
chuhanhandianzishangwu

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$719.00
$799.00
EdgeStar KC2000TWIN Full Size Dual Tap Kegerator & Draft Beer Dispenser - Black
Amazon.com

$172.35
2 Inch Tri Clamp Keg Manifold With Ball Lock Posts, Pressure Gauge, PRV (0-30 PSI) – Homebrew, Fermentation, Kegging System
wuhanshijiayangzhiyimaoyiyouxiangongsi

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$28.98
Five Star - 6022b_ - Star San - 32 Ounce - High Foaming Sanitizer
Great Fermentations of Indiana

$44.99
$49.95
Craft A Brew - Mead Making Kit – Reusable Make Your Own Mead Kit – Yields 1 Gallon of Mead
Craft a Brew

$159.99 ($26.66 / Count)
3M High Flow Series System BREW120-MS, 5616001, For Brewed Coffee and Hot Tea, Valve-in-Head Design
SpaceCityProviders

$479.00
$559.00
EdgeStar KC1000SS Craft Brew Kegerator for 1/6 Barrel and Cornelius Kegs
Amazon.com

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
Guangshui Weilu You Trading Co., Ltd
bracconiere
Jolly Alcoholic - In Remembrance 2023
There's a way to titrate your NaOH with citric acid IIRC, which is supposed to be easier to make accurate solutions of. It's described in detail in Dunn's Scientific Soapmaking.
https://www.amazon.com/Scientific-Soapmaking-Chemistry-Cold-Process/dp/1935652095
man, this is an eyeball titration! i mean seriously, you'd have to whip out a mass spectrometer....you still have to trust your vision to tell when it just turns kinda pink! and i did this in water, so doing it in apple juice you might over estimate a bit, before it's visibly notcible....
bracconiere
Jolly Alcoholic - In Remembrance 2023
well i dropped a 20 spot on this malic, and got to thinking about the percentage of the mol weight, so i decided to try my 1g lye solution with more malic, 190mg's...this time it took 22g's of the lye solution...not the 18-19 it should have but i did the molecular math and it worked out....
forgive my stupid mistakes... but i think it's just a matter of doing the math? i don't know how that kit works with out a percentage calculation.....
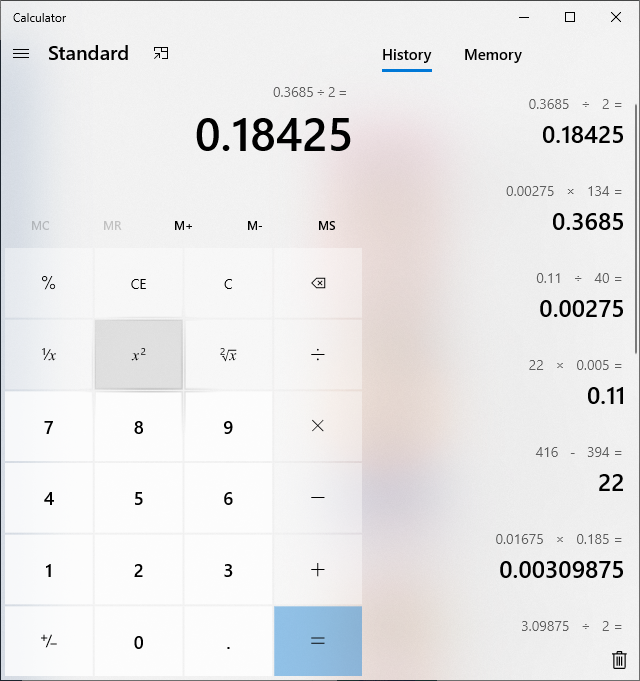
forgive my stupid mistakes... but i think it's just a matter of doing the math? i don't know how that kit works with out a percentage calculation.....

Kees
Well-Known Member
http://www.cider.org.uk/acid_titration.html
Detailed explanation of titration of malic acid, explaning the various points of the titration curve.:
https://socratic.org/questions/calc...0-2m-maleic-acid-with-0-2m-sodium-hydroxide-c
Detailed explanation of titration of malic acid, explaning the various points of the titration curve.:
https://socratic.org/questions/calc...0-2m-maleic-acid-with-0-2m-sodium-hydroxide-c
Last edited:
bracconiere
Jolly Alcoholic - In Remembrance 2023
http://www.cider.org.uk/acid_titration.html
Detailed explanation of titration of malic acid, explaning the various points of the titration curve.:
https://socratic.org/questions/calc...0-2m-maleic-acid-with-0-2m-sodium-hydroxide-c
i think just diluting the lye down a lot, then dividing how many mols of lye it took...then multiplying that by the mol weight of malic acid, and then dividing by 2 is the best way....
lye is 40, malic is 134, and it takes 2 mols of lye to neutrilize 1 mol of malic...
Kees
Well-Known Member
You will need to plot an entire titration curve. You need to work out how many moles of NaOH you have added going from the middle of the first steep leg to the middle of the 2nd steep leg of your curve. That is wil give you the molarity of your juice.
bracconiere
Jolly Alcoholic - In Remembrance 2023
You will need to plot an entire titration curve. You need to work out how many moles of NaOH you have added going from the middle of the first steep leg to the middle of the 2nd steep leg of your curve. That is wil give you the molarity of your juice.
i don't think you need a curve? 1Mol of lye is 39.98 or something, it takes two mols to neutrilize a mol of malic acid, and malic acid has mol weight of 134? you just need to dillute the lye down enough so that you can weigh much you had to add... then do the calculation? how many molcules of malic acid, compared to how many molecules of lye? and malic will take two molecules of lye per molecule?
Mols are like SG for molecules? but with how many of them there are? and how much gravity affects them or something?
i mean lye has a molar weight of ~40...but lead has a mol weight of 207? and if you have ever lift lead you know it's heavy for it's size!
but the calculation, is how many molecules are reacting? one has a weight of 40 per molecule, the other 134....it takes two of the 40 ones to nuetrilize 1 of the 134 one?
Last edited:
Kees
Well-Known Member
Without a curve, how would you know you have neutralized all the H+?i think just diluting the lye down a lot, then dividing how many mols of lye it took...then multiplying that by the mol weight of malic acid, and then dividing by 2 is the best way....
lye is 40, malic is 134, and it takes 2 mols of lye to neutrilize 1 mol of malic...
bracconiere
Jolly Alcoholic - In Remembrance 2023
Without a curve, how would you know you have neutralized all the H+?
phenolphthalein will turn pink?

Kees
Well-Known Member
That will only tell you you have reached a certain pH. It does not tell you how many moles of malic acid you have in your juice.phenolphthalein will turn pink?
There's a lot of confusion here as three entirely different things are treated as one.
1st: how many moles of malic acid does the juice contain? 2nd: how much acid is there in the juice? 3rd how much lye do you need to reach pH=7 (i.e. what is the buffering capacity of the juice)?
As malic acid is the dominant acid in the juice, total acidity is taken to be the malic acid content but that is a simplification of the truth. Even if it were, there are many other molecules (e.g. tartaric acid, citric acid) in the juice that can bind OH- or H+ without changing pH. This is called buffering capacity.
Before you start titrating you need to ask yourself: what are my test results going to tell me? Or, even better: how am I going to find out what I want to find out?
Last edited:
bracconiere
Jolly Alcoholic - In Remembrance 2023
That will only tell you you have reached a certain pH. It does not tell you how many moles of malic acid you have in your juice.
i think i said that, but i can titrate pure malic acid in water with lye, and get a result within a few mg's of what 'i know' is reality?
i thought that's what's trying to be done?
Yep, after reading all this. Definately made my head hurt.
LOL, it's simple
bracconiere
Jolly Alcoholic - In Remembrance 2023
if you ever want to eyeball this stuff....something like this will give you volume per mol...
https://www.aqua-calc.com/calculate/mole-to-volume-and-weight/substance/sodium-blank-hydroxide
https://www.aqua-calc.com/calculate/mole-to-volume-and-weight/substance/sodium-blank-hydroxide
Similar threads
- Replies
- 9
- Views
- 1K
- Replies
- 5
- Views
- 1K































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)








