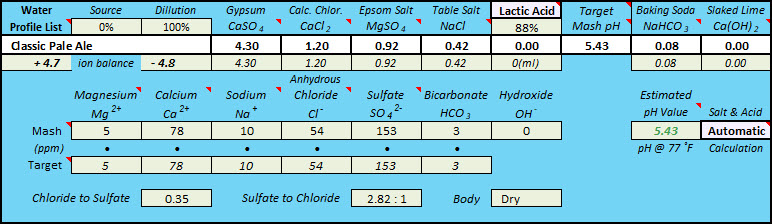
I've read online that people usually need to acidify their mash, especially for lighter beers. However, every time I punch in my salt additions into Bru'n Water, my mash pH is either pretty close to where it should be or actually is a bit too low. In order to get to the right pH, I actually need to add alkalinity to it, using baking soda. I use the Pale Ale profile for most things.
I do a full volume mash, no sparge BIAB, using distilled water (everything is set to zero in Bru'n Water). My process is to tweak additions of gypsum, CaCl, and Epsom to get the right ppms of each ion. For my standard APA/IPA, it's usually around 12g gypsum, 2.5 CaCl, and 5 Epsom. At that point, my pH is usually hovering around 5.2 or so. Then I need to add a bit of baking soda to raise the pH as well as for sodium.
I guess my question here is, why do others normally need to acidify their mash to get to the right pH? Does it make sense that the pH of distilled water after these doses of salts is around where it should be? Also, would canning salt be a better choice for sodium instead of baking soda if the pH is in the right ball park?
I do a full volume mash, no sparge BIAB, using distilled water (everything is set to zero in Bru'n Water). My process is to tweak additions of gypsum, CaCl, and Epsom to get the right ppms of each ion. For my standard APA/IPA, it's usually around 12g gypsum, 2.5 CaCl, and 5 Epsom. At that point, my pH is usually hovering around 5.2 or so. Then I need to add a bit of baking soda to raise the pH as well as for sodium.
I guess my question here is, why do others normally need to acidify their mash to get to the right pH? Does it make sense that the pH of distilled water after these doses of salts is around where it should be? Also, would canning salt be a better choice for sodium instead of baking soda if the pH is in the right ball park?