I’ve been using bru’n successfully for years, but have recently started doing much lighter beers. Like a recent helles, and Pilsner. I’ve realize that when my SRM is under five, my pH seems to be way off. I’ve done other beers in between like IPAs and Marzens and the pH is very close to calculated, but when I do very light beers, they are way off. I’m calculating/shooting for around 5.3, and the mash pH is coming out right around 5.6 for these beers. I use acid malt to adjust pH downward instead of adding acid. Any ideas?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Mash pH off, but only with light beers.?
- Thread starter trapae
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
No ideas as to why because there are a host of things that can go wrong with pH measurement. But, I will say pH 5.6 is where you want to be with a helles anyway! 
Have you re calibrated your meter lately?I’ve been using bru’n successfully for years, but have recently started doing much lighter beers. Like a recent helles, and Pilsner. I’ve realize that when my SRM is under five, my pH seems to be way off. I’ve done other beers in between like IPAs and Marzens and the pH is very close to calculated, but when I do very light beers, they are way off. I’m calculating/shooting for around 5.3, and the mash pH is coming out right around 5.6 for these beers. I use acid malt to adjust pH downward instead of adding acid. Any ideas?
Are you using Rahr 2 row?
That's pre-acidified somewhat to start with. To get around it, need to adjust the color, choose the SRM to be 5 instead of 2.
That's pre-acidified somewhat to start with. To get around it, need to adjust the color, choose the SRM to be 5 instead of 2.
rmr9
Well-Known Member
I’ve had the same thing happen recently with a pils as well. I went down a path of trying to identify where an off flavor was coming from and decided it was time to bust out my pH meter and found the same difference round about as you - should’ve been 5.3 based on the calculator but came to be 5.6 after adding a lot more lactic than was called for. I just took it as something to be mindful of when brewing lighter styles using the calculators.

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$20.94
$29.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.com

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
Guangshui Weilu You Trading Co., Ltd

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$479.00
$559.00
EdgeStar KC1000SS Craft Brew Kegerator for 1/6 Barrel and Cornelius Kegs
Amazon.com

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid MFL)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive
If you're using acid malt to acidify your mash, the first thing I would be concerned with is the strength of the acid malt. The Supporter's version of Bru'n Water has an acid strength factor that you can use to tune the acidity of the acid malt in practice. The free version is purposely striped of extra inputs so that new users aren't overwhelmed with the choices.
In practice with the Supporter's version, input your grain bill and water and then brew and monitor mashing pH. If the pH at around the 45 minute mark isn't close, go back to Bru'n Water and adjust the acid malt strength setting on the Grain Bill Input sheet until the predicted mash pH matches what your late mash pH was measured at. That should significantly improve your targeting.
In practice with the Supporter's version, input your grain bill and water and then brew and monitor mashing pH. If the pH at around the 45 minute mark isn't close, go back to Bru'n Water and adjust the acid malt strength setting on the Grain Bill Input sheet until the predicted mash pH matches what your late mash pH was measured at. That should significantly improve your targeting.
5.6 is actually probably perfect, and 5.3 too low. Depends on the temperature at which you are measuring. Fall down some rabbit holes:
https://www.homebrewtalk.com/threads/will-it-mash-at-ph-5-00.667992/page-2#post-8653242
https://www.homebrewtalk.com/thread...-brewers-do-not-mash-at-5-2-to-5-6-ph.671764/
https://www.homebrewtalk.com/threads/will-it-mash-at-ph-5-00.667992/page-2#post-8653242
https://www.homebrewtalk.com/thread...-brewers-do-not-mash-at-5-2-to-5-6-ph.671764/
I sometimes see a descrepancy with my light colored lager PH also, but weirdly not all the time. I target 5.4 mash pH for my lagers and adjust closer to 5.2 at end of boil if needed, a holdover from when I dabbled with low oxygen brewing and I would say 75% of the time my mash pH is higher, like 5.55 or 5.6, with a calibrated meter. I use lactic for adjustments instead of acidulated malts. However, I have always written off the descrepancy to my water salts, that if say I added 3 grams of Calcium Chloride to reach my pH, because of moisture in it, I may be only adding 1-1.5 grams. I assume the same would apply to other salts too?
I did do a test once, putting 8 grams of CaCL2 in the oven for an hour at 300F and then weighing it again, and after the heat drove off the moisture, I only had 6.2 grams. I should do this test with gpysum and Epsom Salt too one of these days. Because of this, I started using liquid CaCl2 and the Bru'n Water calculator for figuring out strength of it based on the gravity. But I found that my mason jar kept getting mold on the lid, so got away from using liquid.
I did do a test once, putting 8 grams of CaCL2 in the oven for an hour at 300F and then weighing it again, and after the heat drove off the moisture, I only had 6.2 grams. I should do this test with gpysum and Epsom Salt too one of these days. Because of this, I started using liquid CaCl2 and the Bru'n Water calculator for figuring out strength of it based on the gravity. But I found that my mason jar kept getting mold on the lid, so got away from using liquid.
Dave, I don't find that 5.6 is ideal for pale beers. That extra couple of tenths does bring out a little more tannin into the finished beer and I'm not a fan of that. In addition, the higher starting work pH means that the yeast have to work harder to acidify the wort into a safe beer pH. And the final reason I find it too high is that I consistently find that producing a beer pH in the 4.2 to 4.4 range helps them present as more crisp and refreshing.
BrewnWKopperKat
ʘ‿ʘ
@IslandLizard : with regard to
consider
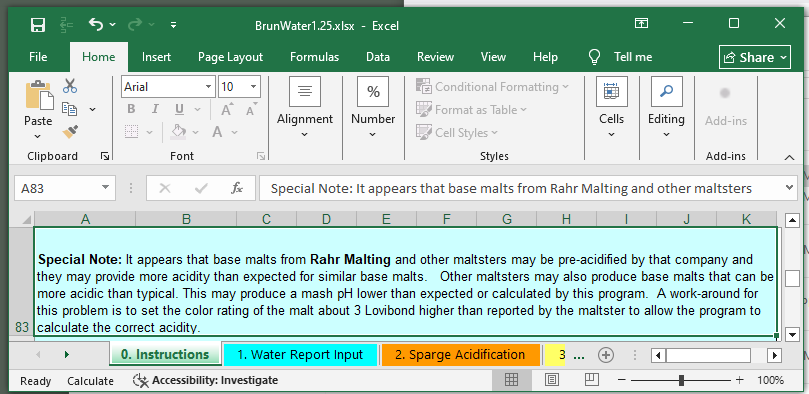
I have a new local home brew store (physical + online ordering) that offers Rahr at an attractive price - so I may want to "shake the dust off" on the pH meter. eta: and perhaps move to the paid version of the spreadsheet.
Are you using Rahr 2 row?
That's pre-acidified somewhat to start with. To get around it, need to adjust the color, choose the SRM to be 5 instead of 2.
consider
- r/Homebrewing/comments/7udi3t/rahr_2row_preacidulated/
- https://www.thebrewingnetwork.com/forum/viewtopic.php?t=30072

I have a new local home brew store (physical + online ordering) that offers Rahr at an attractive price - so I may want to "shake the dust off" on the pH meter. eta: and perhaps move to the paid version of the spreadsheet.
I think it is important to clarify that a mash pH of 5.6 should be accompanied by an acid addition at say 15 minutes left in the boil to drop the wort down to pH 5.1-5.3 Not only does this help the yeast but also aids in things Whirlfloc to work better.Dave, I don't find that 5.6 is ideal for pale beers. That extra couple of tenths does bring out a little more tannin into the finished beer and I'm not a fan of that. In addition, the higher starting work pH means that the yeast have to work harder to acidify the wort into a safe beer pH. And the final reason I find it too high is that I consistently find that producing a beer pH in the 4.2 to 4.4 range helps them present as more crisp and refreshing.
Also, a pH of 5.6 helps keep SMM in check so there is less chance of it turning into DMS. The slightly higher pH seems to keep certain chemical processes more at bay than lower pH numbers. Kunze is a big fan of higher pH levels for pale beers as many prominent German brewers are. I agree with the idea of keeping the pH higher for the mash & most of the boil then lowering it before the yeast get involved.
Similar threads
- Replies
- 15
- Views
- 971
- Replies
- 13
- Views
- 845
- Replies
- 64
- Views
- 4K
- Replies
- 61
- Views
- 4K











![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)


































