DuncB
Well-Known Member
I do purge the starsan into a bucket after the fermenting keg has purged it's own headspace. I do this starsan into a spare container.
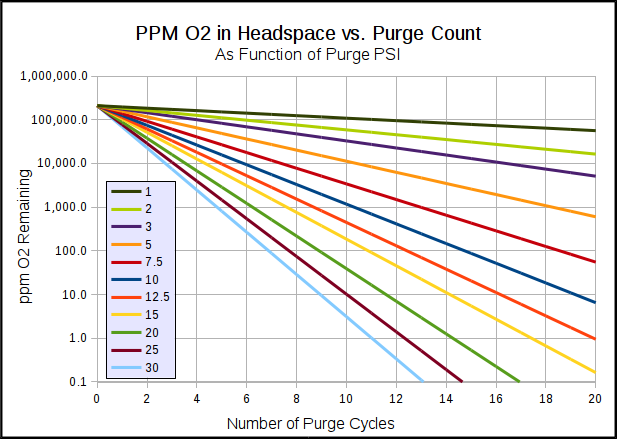
Yes dilution works but I'm trying to limit the oxygen as much as possible so I have 4 litres of oxygen less to dilute. I suppose the keg being flushed doesn't have to be in the keg ferment ( chest freezer).
Yes dilution works but I'm trying to limit the oxygen as much as possible so I have 4 litres of oxygen less to dilute. I suppose the keg being flushed doesn't have to be in the keg ferment ( chest freezer).




![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)