He's making it sound a lot more complex than it is.
It's really not complex at all though it may sound so. I've got all the gear including a blender and have decided that it's just easier not to fiddle with that stuff but rather just adjust the CO2 pressure to serve or store/carbonate. Whether you or anyone else finds it so is, of course, dependent how you feel after you have tried it. If it sounds too complex keep it in mind anyway as you can use it in a pinch if you run out of beergas.
The significantly higher pressure with Nitro (30psi vs. 10psi) definitely has an effect.
Yes it does. Sparkler plates don't work very well at 10 psig. But it doesn't matter what you push the beer with as long as it is properly carbonated (about 1 vol) and the push gas is effectively inert. Nitrogen is cheap and readily available but argon or helium would do. CO2 also works fine because that's already in the beer and in an hour or 2 it isn't going to pick up much more at 30 psig relative to its 1 vol level.
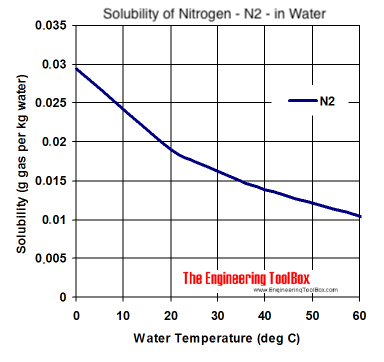
First - Nitrogen does dissolve in water at approximately .0138g/L at 1 bar.
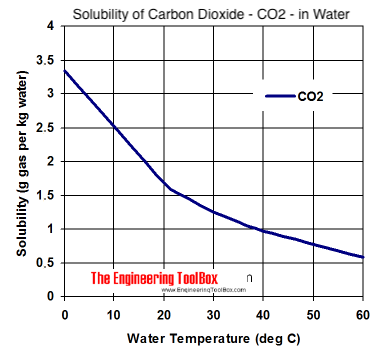
Taking that number as correct that amounts to .0138/28 = half a millimole which is approximately .01 liter i.e. its solubility is 0.01 vols. The solubility of CO2 at 1 bar is about 1 vol - 100 times greater than than of N2.
CO2 dissolves at .0089g/L at 1bar (source,
http://www.engineeringtoolbox.com/air-solubility-water-d_639.html ) That's nearly 1.5x the rate of CO2.
Read the page carefully. That the solubility of oxygen. The solubility of CO2 is about 100 times that of N2. Use common sense. If you put some water in a saucepan and heat it does it 'fizz' with vigor in any way comparable to the fizzing of a low volumes ale put in saucepan and warmed?
Second, it's creamier partly because N2 bubbles are significantly smaller than CO2 bubbles.
So small they don't exist! Or not in any appreciable number. Supposing that you pressurize with 2 bar absolute N2 and 1 bar absolute CO2 (45 psia, 30 psig) given the 100x solubility ratio of CO2 the nitrogen bubbles, if they even formed, would be at most 2% of the total bubbles.
Injecting air into a glass (air being 80% nitrogen) may get you bubbles, but really, you're only releasing the CO2 - not injecting N2 into solution and getting the very persistent, very tiny, very thick bubbles that you get on a real Nitro system.
Probably the best way to demonstrate the fallacy of this statement would be to draw a pint of English ale from an engine equipped with a sparkler. I think we have lost sight of the fact that all Guiness's research on mixed gas and widgets and on the near universal use of beer gas in pubs in the UK was done in order simulate the foam on a glass of sparkler drawn ale without having to deal with the capital costs and maintenance of the traditional hand pumps.
On Wednesday, I will try to post pics of the same beer, same batch, one keg served on Nitro, one keg served on CO2. There is a definite difference. I'd do it from home, but I don't have two kegs of the same brew to demonstrate (not right now, anyway. . .)
Be sure to include one of a pint drawn through an engine.
Third, the APPARENT taste is slightly sweeter on Nitro. Why? Well, CO2 does dissolve in water, but it changes to Carbonic Acid (H2CO3(aq)) albeit only about 16% of the CO2 does so. But it has an effect on our taste - we taste the slight increase in bitterness.
There is just as much CO2 in properly conditioned stout (about 1 vol) whether it is pushed by an engine through a sparkler or nitrogen or CO2 through a sparkler plate. Thus any difference in taste when pushed by different means cannot be explained in terms of the CO2.
If you take distilled water (pH 7) and carbonate it at 1 bar, it will have a pH of about 5.7 and will taste distinctly sour or bitter.
At a bar it would actually be under 4.
The reason we like carbonated beverages has little to do with the pH of the beer. It is the bursting of the bubbles on our tongues which release CO2 close to nerve endings. That CO2 forms carbonic acid in/on (?) the cells which stimulates a pain response. Small enough and far enough apart these pain responses are pleasurable. This comment is based on my shaky memory of an article I read a couple of years ago about which I have no more specifics.
Nitro CAN displace some of that CO2 and thus make a smoother and slightly sweeter (apparent) taste.
As noted above based on the gas laws nitrogen does not
displace anything. It is its physical effect on properly carbonated beer that gives the lovely creamy head. The reason the creamy head is nice is because of its texture. But drinkers knew that before anyone ever experimented with driving beer with mix. They knew that when it was driven by the arm of a sturdy lass pulling the handle on a beer engine.
What does nitrogen taste like?


















![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)