dunbruha
Well-Known Member
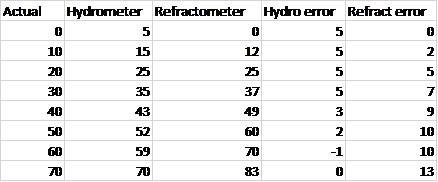
So I brewed my first batch yesterday, and took measurements of my gravities at various times throughout the process. I used a hydrometer and a refractometer (an ATC model with specific gravity markings directly on the readout). I noticed that the readings were very different between the two instruments. So I decided to make a set of standard solutions of DME at various gravities (0, 10, 20, 30, 40, 50, 60, & 70) and see what values each method gave me. The graphs and the data are attached below. (The hydrometer readings are corrected for temperatureeverything was at 72F.)
You can see that the hydrometer was 5 points too high from plain water to 1.030, and then the error started to shrink, getting to pretty much spot on at 1.070.
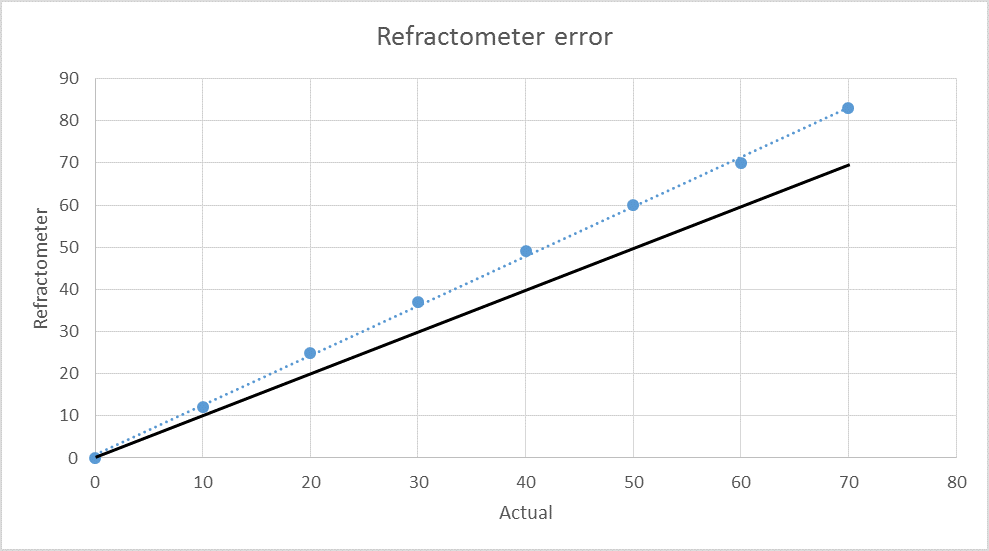
In contrast, the refractometer started out correct in plain water, but the error increased in an increasing rate up to 1.070.
Is there any way that I can apply a correction factor to these graphs? Neither one has a constant error, and my math skills arent good enough to figure out what to do. Id appreciate any ideas!

You can see that the hydrometer was 5 points too high from plain water to 1.030, and then the error started to shrink, getting to pretty much spot on at 1.070.
In contrast, the refractometer started out correct in plain water, but the error increased in an increasing rate up to 1.070.
Is there any way that I can apply a correction factor to these graphs? Neither one has a constant error, and my math skills arent good enough to figure out what to do. Id appreciate any ideas!


























































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)