Thanks for responding, it looked like this site was slow to update during the holidays so I gave up on this thread. Sorry.
-"What's in the first picture?"
It was the same picture.
-"Also, what solvent system?"
0.002 N H2SO4
-"Do you suspect the maltodextrin coeluted with other unfermentables?"
I really don't think there was much in the way of unfermentables in this particular finished beer besides the maltodextrin I added, I'm chemist A in this scenario.
-"Therefore, could both hypotheses have value, ie. poor conversion and added unfermentables with similar molecular properties?"
YES!! That is the answer I am looking for, and I should add that I forgot to mention the other half of the story. A buddy of mine started all-grain brewing, in his first batch he let the mash temp get away from him and so he expected an inefficient mash(I don't have a copy) but his chromatogram looked very similar to mine with almost the same amount of "highers", only he DID NOT add any maltodextrin. He knew for a fact his mash temp. was inappropriate, and I know mine was appropriate. So in the case of my buddies beer I would agree with chemist B, but in my case I think that my hypothesis is most correct.
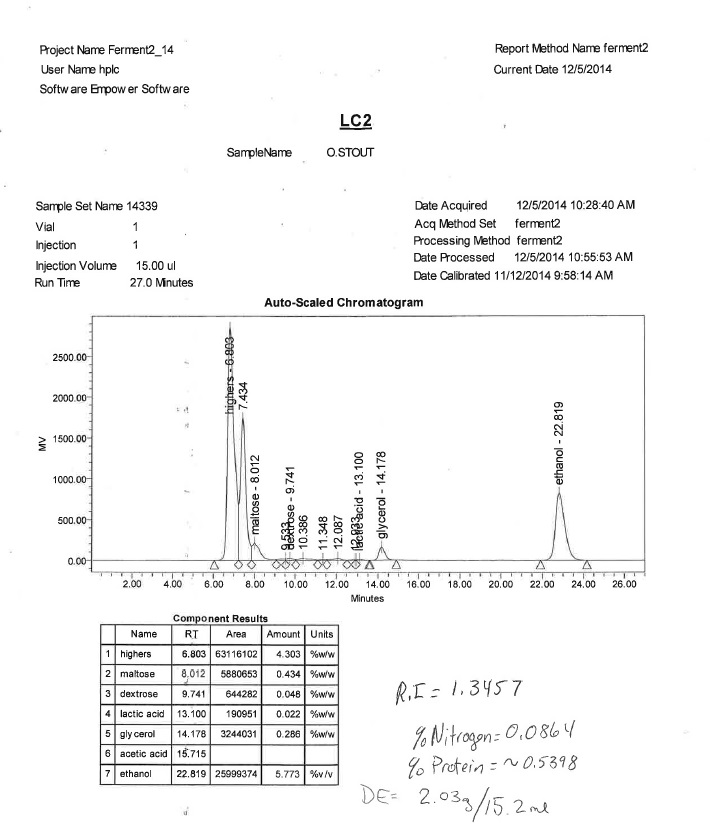
-"Do you suspect the peak at 7.434 to be maltotriose?"
Not necessarily, but I can see where you would expect that based on the elution time, I did add maltodextrin.
-"As an aside...where did the lactic acid come from?"
Perhaps LME...this was a partial mash.
Thank you for responding, your brief comments seem to be confirming my line of thinking...
I will have to go ahead and disagree to some extent with this statement though...
"Maltodextrin is not a "non-preferential fermentable," is it in fact unfermentable by S. cerevisiae."
Though I admit my 'non-preferential fermentable' may not have been the best choice of words, in regards to the fermentability of maltodextrins by S. cerevisiae it isn't so black and white per se. But I definetly get what you are saying on a superfiacial level maltodextrin is for the most part not fermentable by S. cerevisiae The degree of fermentability of maltodextrin would depend on the DP profile of the particular maltodextrin and the process to which it was produced, as well as the DE of the maltodextrin. Maltodextrin has many different polymer lengths to include DP1s and DP2s which would be preferentially utilized by S. cerevisiae, it takes a different HPLC program to determine the particular DP profile.
I have seen literature somewhere with figures of 12-16% fermentability, don't remember the source, but again it would depend on the DP profile of the particular maltodexrin. I should add, this was a 10 DE maltodextrin.
Anyways, thank you again for responding...this is thought provoking.


![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)








