First, I appreciate the time you took to explain this all!
Not a problem as I'd been thinking along these lines as a result of responding to a similar post at
https://www.homebrewtalk.com/showthread.php?t=575486
Aside from carbonation, is the CO2 only lowering the pH in this?
In a natural water in equilibrium with limestone the pH, alkalinity ad hardness are all set by the amount of CO2 to which the water is exposed. This CO2 in mesic region groundwater is produced by respiring soil bacteria.
Therefore knowing the alkalinity and the pH one (not me, but someone) can calculate the amount of CO2 present?
Yes and you can do it too. The math is at
https://www.homebrewtalk.com/showthread.php?t=473408. You can also get it from a couple of charts (obviously based on that math) which I'll post tomorrow.
If that is correct, assuming "enough" carbonation, is it reasonable to assume the carbonation level is less important to the taste since the carbonation of the drink changes anyway in the glass?
I can't comment on what is 'enough'. That's a matter of personal taste. The water in question is described a sparkling but apparently only contains 0.1 atm of CO2. That's not very 'sparkling' to my way of thinking. [After correcting the magnesium level it appears to contain CO2 at about half an atmosphere - more sparkly for sure but still not very] There are two aspects to CO2. One is the pain (and I'm told that's what it actually is) caused by carbonic acid attacking the nerves in your tongue and the other is the tartness caused by the low pH of waters in which a lot of carbonic acid is dissolved. Thus the carbonation level is very important to the way the beverage is perceived and yes, it changes dramatically as CO2 escapes over the time it takes to finish the drink. Same as with beer!
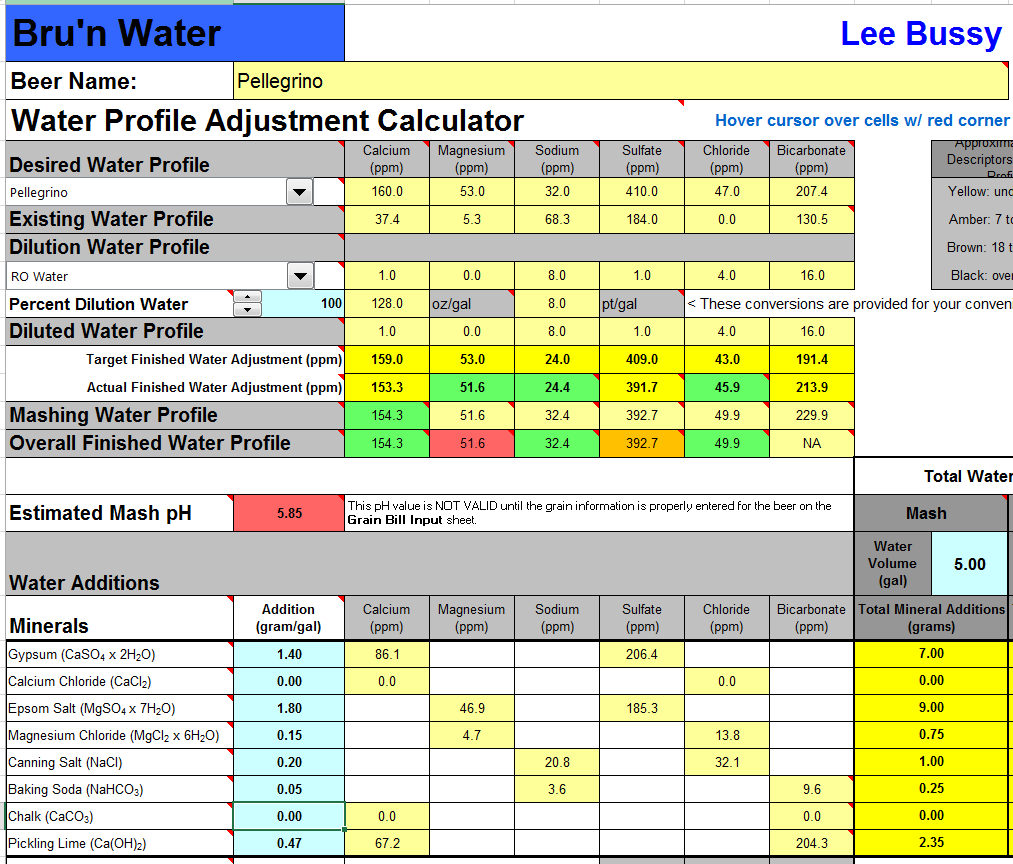
Magnesium seems about a third or less of what it should be ... unless I'm mmissing something? To be fair what I am doing to make sense in my small brain is multuplying those numbers by 3.78541 to get mg/gal and then dividing by 1000 to get g / gal and then plugging them in Martin's spreadsheet.
Well I'm afraid the reason the Mg is so low is because I fat fingered (or, to be really honest about it, more probably fat-brained) 15 mg/L into the Mg++ field instead of 53 mg/L. I'll go back and edit the earlier post for the RO water case tonight and will fix the rest of the numbers tomorrow. In any case, the new MgSO4.7H2O requirement is 537.4 mg/L.
I know if I spend enough time trying to figure it all out I *might* be able to make my own spreadsheet and understand it more.
It is clearly pretty simple to put together a spreadsheet that figures out how many mg of, for example, Mg and SO4= one gets from x mg of MgSO4.7H2O by looking up the molecular weights of Epsom salts, water, magnesium and sulfuric acid (subtract 2 to get molecular weight of sulfate ion). Where it gets a little tricky is in handling the carbonic acid system but that is really not that bad as you'll see if you look at
https://www.homebrewtalk.com/showthread.php?t=473408 and add
Step 12: CO2 = C*f0*44; H2CO3 = C*f0*62 (grams/L). Once you have a spread sheet that calculates Mg++, SO4=, HCO3-, CO3=, Cl-, Ca++ etc. from amounts of added H2SO4, HCl, NaCl, HLac, CO2, NaHCO3, MgSO4 etc
AT A SPECIFIED pH it is a simple matter to add in a list of desired ion concentrations, compute the logs of the absolute values of the differences between calculated and desired, compute the sum of the squares of those errors and ask Solver to pick salt amounts that minimize that sum of squares. I absolutely guarantee that you will have a deeper understanding of how this all works if you pull this off. Don't hesitate to ask for help.
For right now the best way I can try to understand it is to use two (or more) different tools and make them agree.
If a tool purports to allow you to synthesize water by adding salts to RO and it doesn't ask you what pH you want the synthesized water to be at then be wary of that tool. If you add neutral salts to water at some pH the pH will stay the same but if you add acid, base, bicarbonate or carbonate salts to water the pH will shift and this must be accounted for. I cannot find where to put the target pH into Bru'n water, for example, and thus cannot figure out how it gives answers if, for example, sodium bicarbonate is added unless it assumes that the bicarbonate ion stays as bicarbonate which it does not do except approximately at pH 8.4.