I'm not sure if anyone has sent one off to Ward and/or posted the results but I promised to do so myself and the numbers are in. Quite frankly, my water knowledge is fairly limited but these numbers seem to be off the charts in several respects. Hopefully our resident water experts will have a look and let us know what they think. What I can say is that the video showing the brew log from the 1000th brew was not intended to misdirect. He was in fact aiming for incredibly hard water.
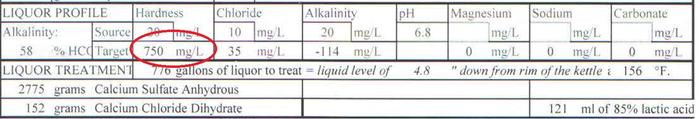
pH - 4.3
TDS - 1584
Electrical Conductivity, mmho/cm - 2.64
Cations/Anions, me/L - 36.6/20.6**
ppm
Sodium, Na - 25
Potassium, K - 802
Calcium, Ca - 110
Magnesium, Mg - 113
Total Hardness, CaCO3 - 746
Nitrate, NO3-N - 17.6
Sulfate, SO4-S - 156
Chloride, Cl - 339
Carbonate, CO3 - <1.0
Bicarbonate, HCO3 - <1
Total Alkalinity, CaCO3 - <1
Total Phosphorus, P - 278.10
Total Iron, Fe - 0.37
"<" = Not Detected/Below Detection Limit
** = Total phosphorus not included in cation/anion calculation
pH - 4.3
TDS - 1584
Electrical Conductivity, mmho/cm - 2.64
Cations/Anions, me/L - 36.6/20.6**
ppm
Sodium, Na - 25
Potassium, K - 802
Calcium, Ca - 110
Magnesium, Mg - 113
Total Hardness, CaCO3 - 746
Nitrate, NO3-N - 17.6
Sulfate, SO4-S - 156
Chloride, Cl - 339
Carbonate, CO3 - <1.0
Bicarbonate, HCO3 - <1
Total Alkalinity, CaCO3 - <1
Total Phosphorus, P - 278.10
Total Iron, Fe - 0.37
"<" = Not Detected/Below Detection Limit
** = Total phosphorus not included in cation/anion calculation












![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)