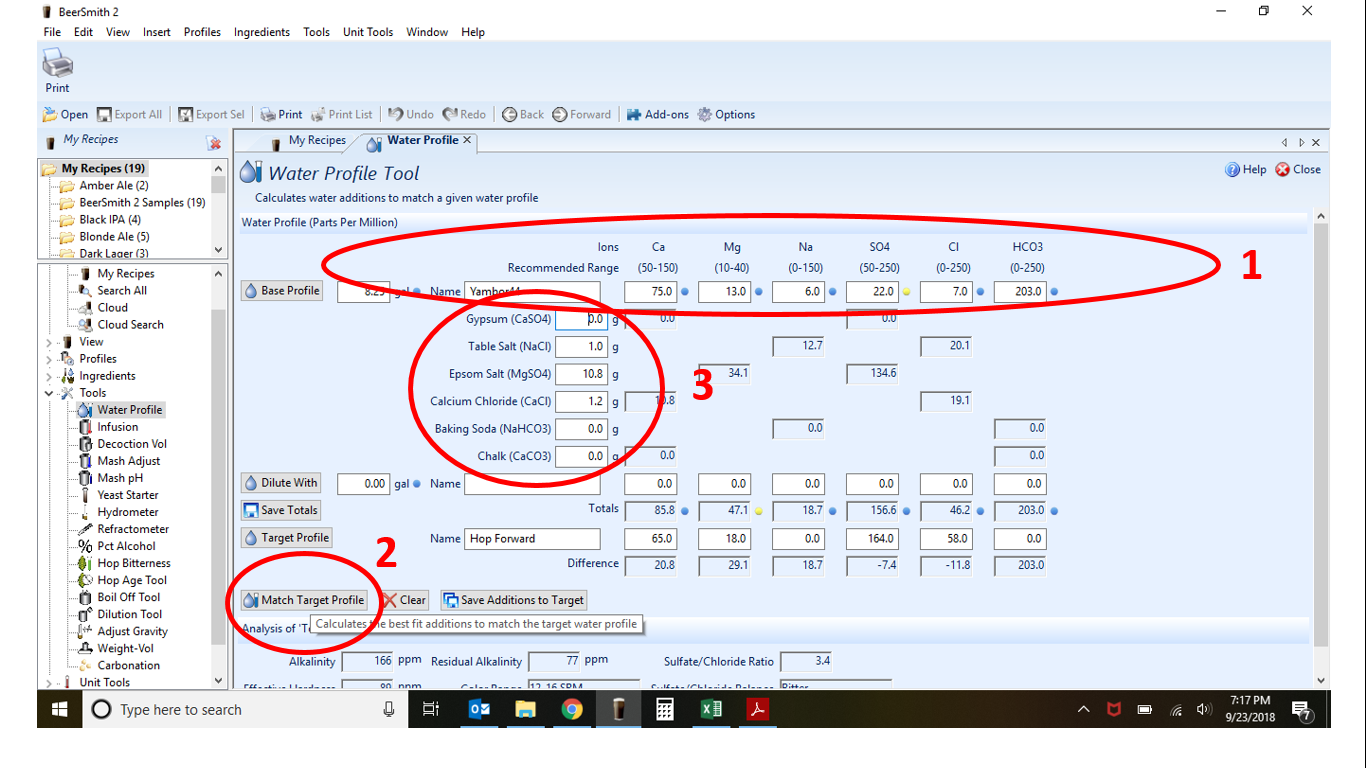
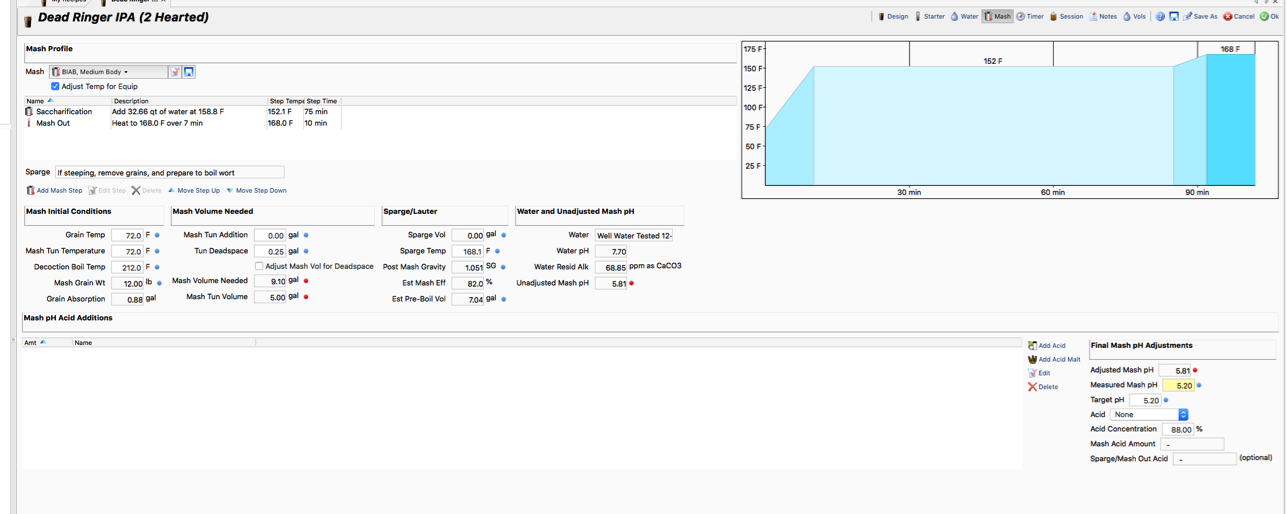
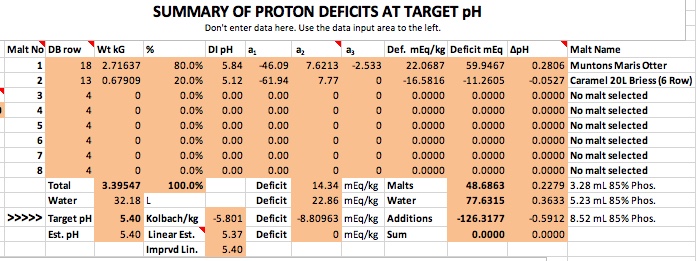
I've been doing some research on water profiles until I am dizzy with it. Long story short I need some input on my Wards Labs water analysis report. Do I need to add a little gypsum and if so, how much and when do I add it?
I recently got back into brewing and purchased a High Gravity BIAB system, 4500 watt system. I pretty much only brew IPA's and right now 5 gallon finished. I start with about 8-8.5 gallons of water depending on the grain bill.
Thank you for any assistance you can offer! - Rob
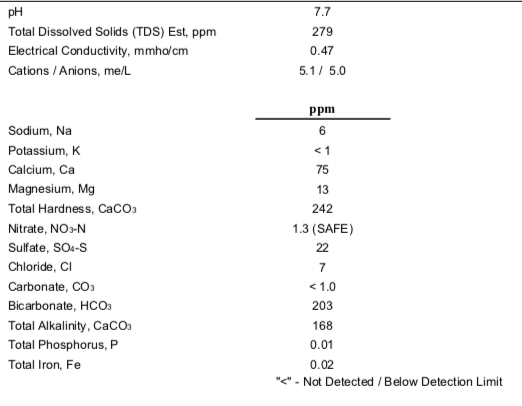
I recently got back into brewing and purchased a High Gravity BIAB system, 4500 watt system. I pretty much only brew IPA's and right now 5 gallon finished. I start with about 8-8.5 gallons of water depending on the grain bill.
Thank you for any assistance you can offer! - Rob