oljimmy
Well-Known Member
Hi All,
I'm doing a BIAB dry stout, standard bill: 62.5% Maris Otter, 25% flaked barley, 12.5% roasted barley. 4.3 gals of spring water with 1 gal of distilled, no sparge. This is my water addition plan so far, with questions below:
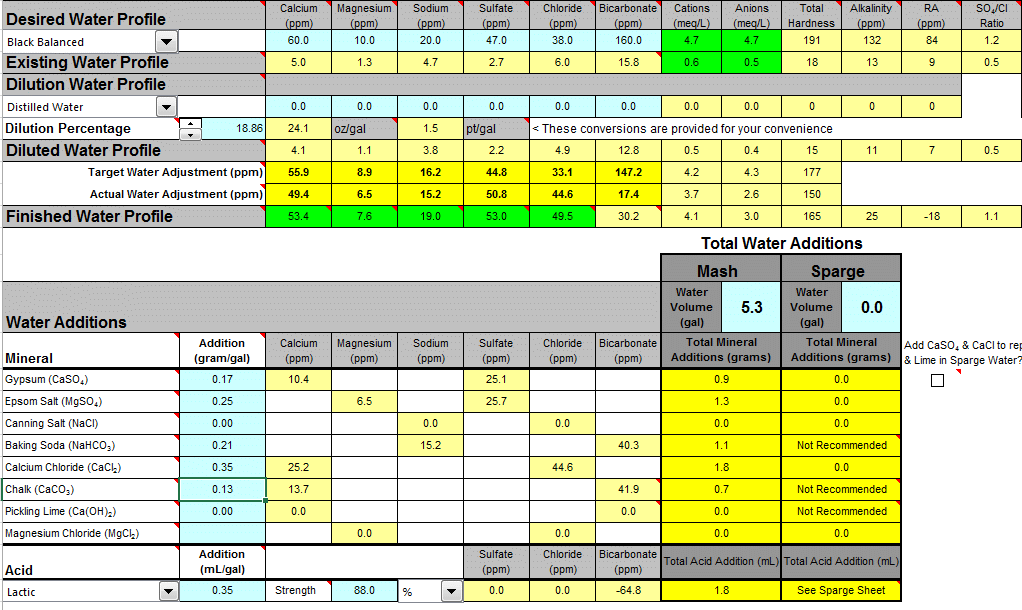
Projected room temp pH is 5.5.
1. Basically, I'm wondering what my priorities should be. I've seen people on here saying that chalk is really hard to dissolve properly, but if I use either calcium chloride or gypsum to up my calcium content, then my sulfate and chloride levels go even higher, and they're already significantly above the style.
2. People have also mentioned that I shouldn't be adding bicarbonate AND acid, but given what I have available (no lime or canning salt) I have a hard time seeing how I can get the minerals I want and not raise my pH to 5.8-5.9. Hence the lactic acid. Should I just suck it up and find some pickling lime and canning salt?
3. Generally, if you had to prioritize minerals, which are most important and which are least important for a dry stout?
Thanks, and sorry for the probably confused questions.
I'm doing a BIAB dry stout, standard bill: 62.5% Maris Otter, 25% flaked barley, 12.5% roasted barley. 4.3 gals of spring water with 1 gal of distilled, no sparge. This is my water addition plan so far, with questions below:

Projected room temp pH is 5.5.
1. Basically, I'm wondering what my priorities should be. I've seen people on here saying that chalk is really hard to dissolve properly, but if I use either calcium chloride or gypsum to up my calcium content, then my sulfate and chloride levels go even higher, and they're already significantly above the style.
2. People have also mentioned that I shouldn't be adding bicarbonate AND acid, but given what I have available (no lime or canning salt) I have a hard time seeing how I can get the minerals I want and not raise my pH to 5.8-5.9. Hence the lactic acid. Should I just suck it up and find some pickling lime and canning salt?
3. Generally, if you had to prioritize minerals, which are most important and which are least important for a dry stout?
Thanks, and sorry for the probably confused questions.


