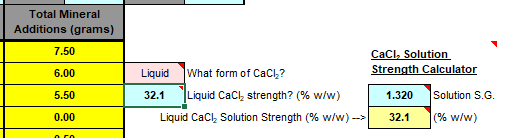worlddivides
Well-Known Member
What are these? I've never seen any brewing website sell anything EXCEPT just "Calcium chloride" without any specification. I'm assuming this is "anhydrous," but if that's the case, what's the "dihydrate"? I've always just assumed calcium chloride is calcium chloride, partially because I've never seen a brewing store selling anything other than just "calcium chloride" without any further specification, but different water profile software will calculate the brewing salts to add with "calcium chloride (anhydrous)" and "calcium chloride (dihydrate)" as completely different things, and I don't want to just add "calcium chloride" to something where I'm supposed to add one and it not be the other.
In the past I would buy RO water and adjust the water profile with brewing salts, but I'm far from an expert on the subject. While I feel like a chef when I come up with beer recipes with grains, hops, yeasts, and so on, when it comes to water chemistry, I just do whatever the program says. In the past I mainly just did whatever Bruin Water gave me. This time, I'm using Brewer's Friend's water profile calculators. I can't remember whether Bruin Water separated calcium chloride into anhydrous and dihydrate or not, but if it did, I probably just used the brewing "calcium chloride" for both without noticing the difference at all.
I looked online, but only a bunch of technical scientific websites came up without any clear explanation (I probably just don't know the right way to search).
In the past I would buy RO water and adjust the water profile with brewing salts, but I'm far from an expert on the subject. While I feel like a chef when I come up with beer recipes with grains, hops, yeasts, and so on, when it comes to water chemistry, I just do whatever the program says. In the past I mainly just did whatever Bruin Water gave me. This time, I'm using Brewer's Friend's water profile calculators. I can't remember whether Bruin Water separated calcium chloride into anhydrous and dihydrate or not, but if it did, I probably just used the brewing "calcium chloride" for both without noticing the difference at all.
I looked online, but only a bunch of technical scientific websites came up without any clear explanation (I probably just don't know the right way to search).





















![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)