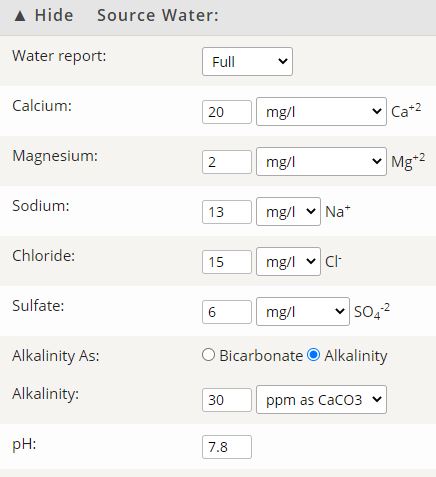
Tonight I got water ready for a porter to be brewed tomorrow, and was taste testing my additions. I did only small amounts in a cup of water just to see how they worked. They were a little concentrated but not terribly so. Most dissolved in the water and weren't very noticeable.
When I got to the Slaked lime however, it was, well, nasty. And it wouldn't dissolve. Is this normal? I am skipping adding it this brew session until I figure out what the deal is with it. I know people add it, EZ Water has a spot for it, and I wasn't planning on much - 2 grams for a 5 gallon batch.
This is what I bought, should I actually go ahead and use it? https://www.amazon.com/gp/product/B074B8F2BP/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1
When I got to the Slaked lime however, it was, well, nasty. And it wouldn't dissolve. Is this normal? I am skipping adding it this brew session until I figure out what the deal is with it. I know people add it, EZ Water has a spot for it, and I wasn't planning on much - 2 grams for a 5 gallon batch.
This is what I bought, should I actually go ahead and use it? https://www.amazon.com/gp/product/B074B8F2BP/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1


















![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)