North_of_60
Well-Known Member
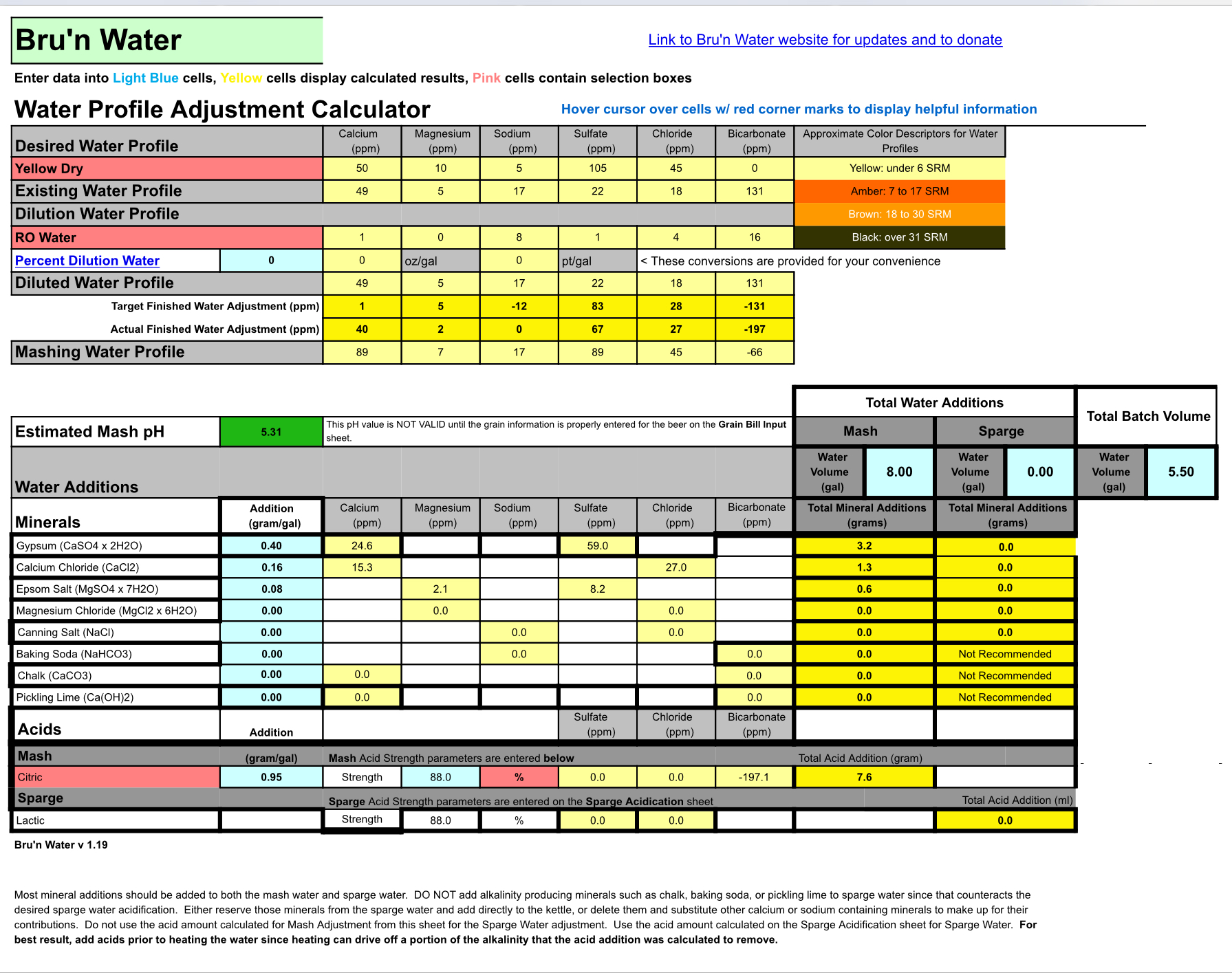
I used the data from the Fort Worth utilities web site for my existing water profile. The latest data is from 2016 and its spread across a large are so the accuracy is probably poor.
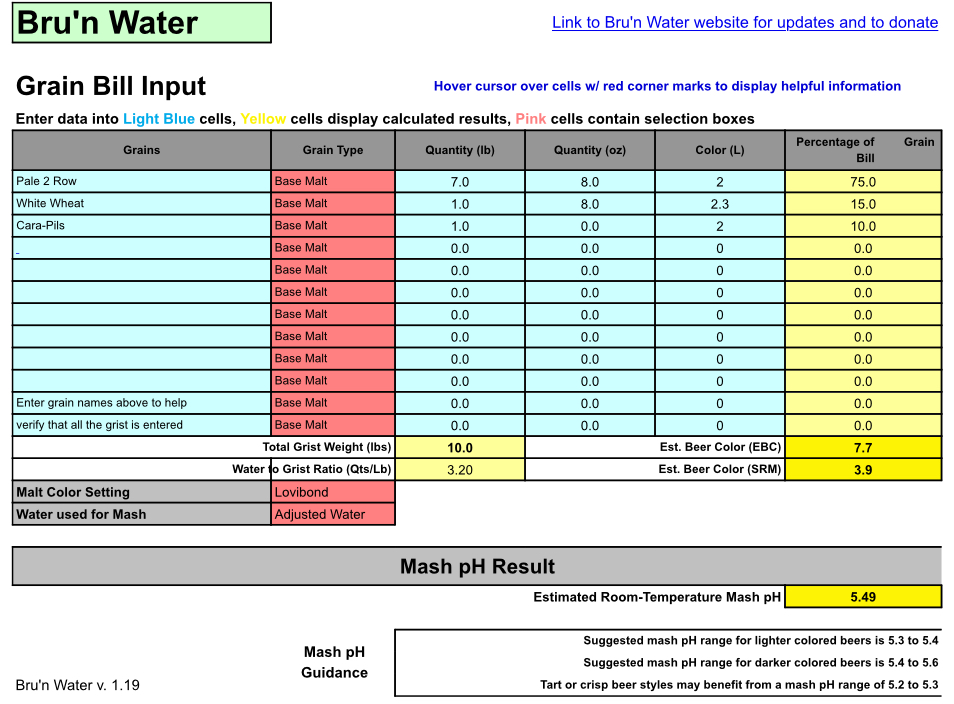
The grain bill is for a Blond Ale 5 gallons batch BIAB 8 gallons mash
1.53 OG
20.2 IBU
5.8% ABV
3.9 SRM
Bru’n Water gave my existing water an untreated pH of 5.98
After the grain bill it changed to 5.95
After additions it lowered to 5.49
To get a lower pH using Acid I get a negative Bicarbonate number.
If I lower the pH using salts the other numbers get high.
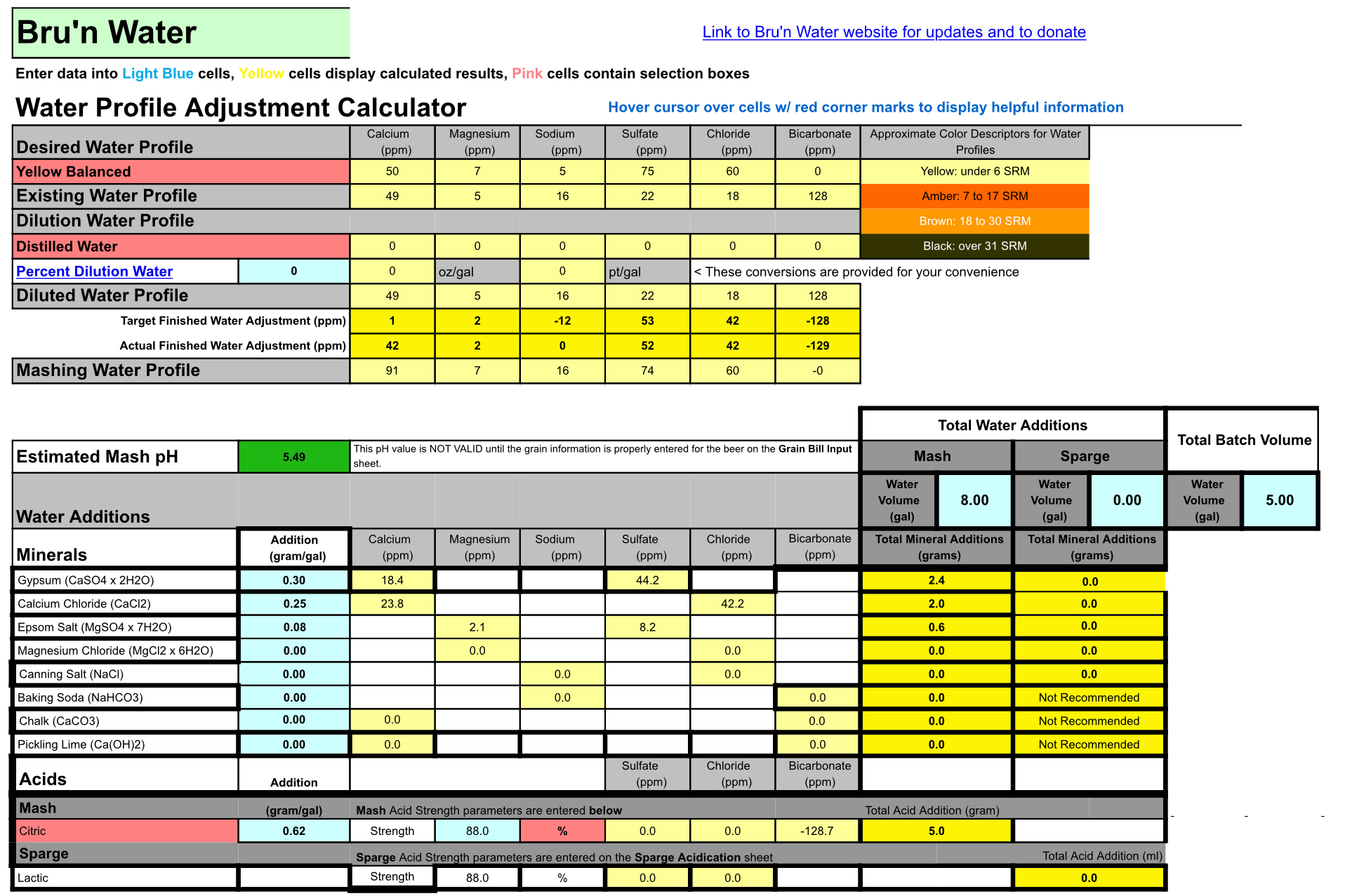
The grain bill is for a Blond Ale 5 gallons batch BIAB 8 gallons mash
1.53 OG
20.2 IBU
5.8% ABV
3.9 SRM
Bru’n Water gave my existing water an untreated pH of 5.98
After the grain bill it changed to 5.95
After additions it lowered to 5.49
To get a lower pH using Acid I get a negative Bicarbonate number.
If I lower the pH using salts the other numbers get high.














![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)