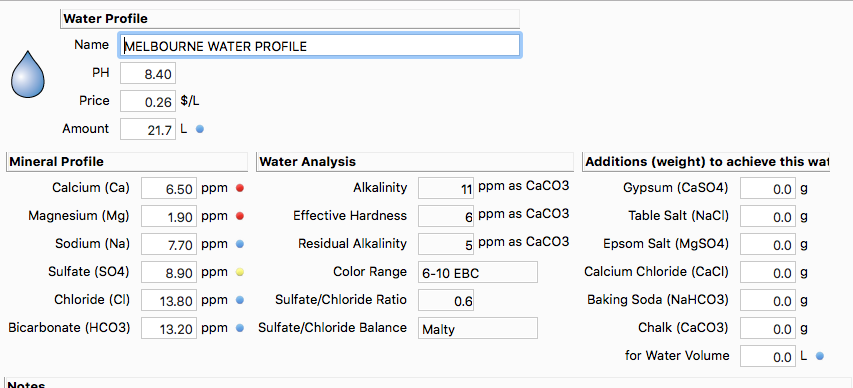
Hi guys, Ive been using the water tools in Beersmith but what I dont get is it tells me to add baking soda to the mash, but then my PH is too high and I have to use lactic acid to reduce it.
Todays beer is shown below. 5 mins after mash in my ph was 5.9 so I added 8mls of lactic to 20 litres water. Its as still a bit high 5 mins later so I added another 4 mls. I should have waited longer as now my mash is 5.12, but from what Ive read thats still ok. However I dont get why beer smith says to add baking soda if Im only going to have to lower the ph again with lactic. Should I just start laving the Baking soda out?

Todays beer is shown below. 5 mins after mash in my ph was 5.9 so I added 8mls of lactic to 20 litres water. Its as still a bit high 5 mins later so I added another 4 mls. I should have waited longer as now my mash is 5.12, but from what Ive read thats still ok. However I dont get why beer smith says to add baking soda if Im only going to have to lower the ph again with lactic. Should I just start laving the Baking soda out?
















































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)










