Protos
Die Schwarzbier Polizei
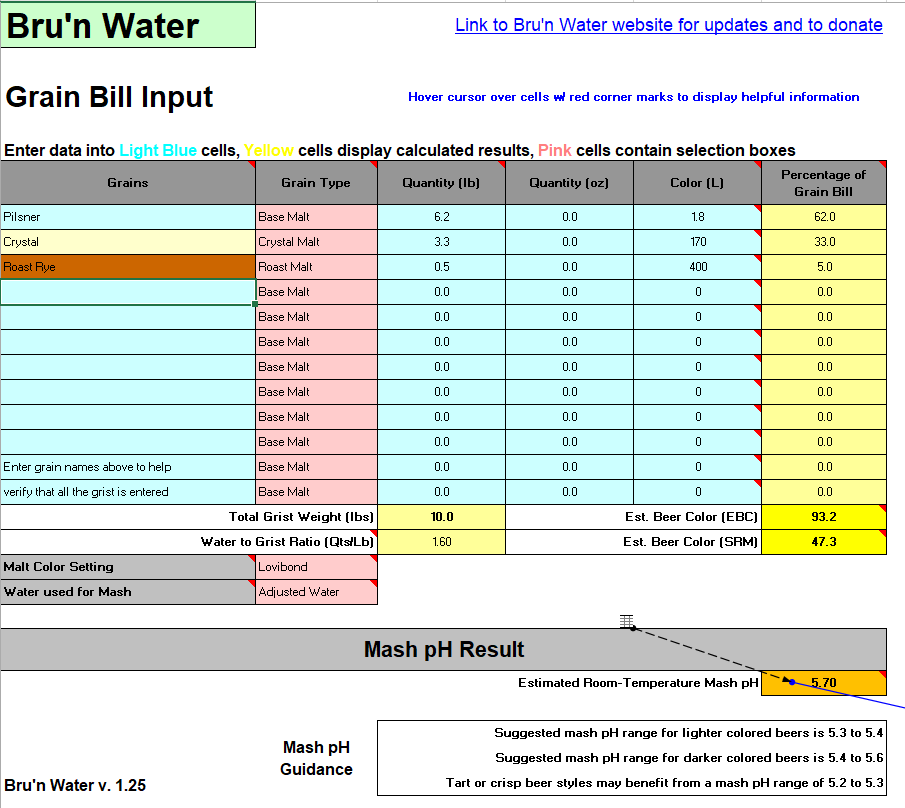
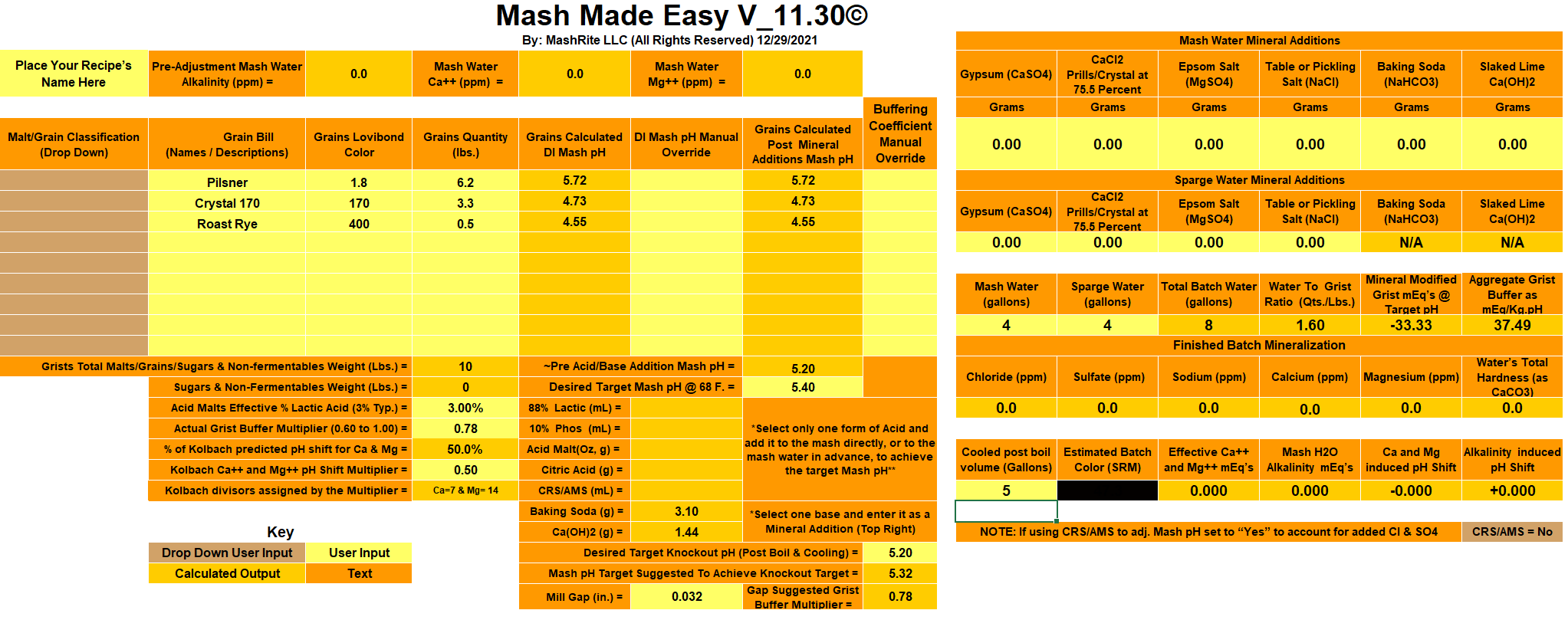
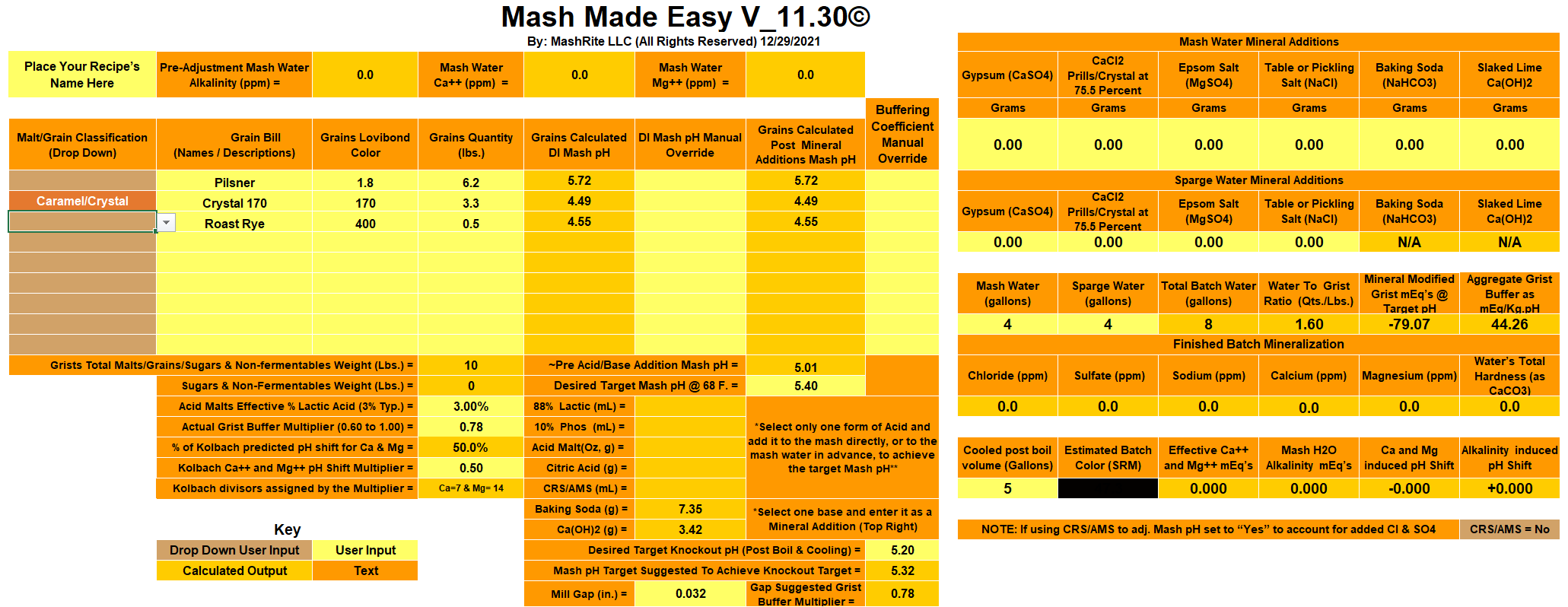
Gentlemen, I'm recreating a historical recipe (a 1920s Polish Baltic Porter) which uses Dark Crystal malt as the third of the grist. Which is unusual, still it's documented in the recipe. The grist is Pilsner 62%, Dark Crystal (170L) 33%, Roasted Rye (400L) 5%. Unfortunately, there's no hints on what kind of water they had and how did they treat it.
Starting with my untreated tap water of circa 270 ppm Bicarbonate, I'm getting an extremely low mash pH prediction of 3.50 in Brun Water. Further calculations show that upping the pH to 5.5 first with Baking Soda (stopping at going past 60 ppm Na) and then with Pickling Lime would result in insane Bicarbonate level of circa 1640 ppm.
Even if I substitute the Dark 170L Crystal with the Medium 60L, the HCO3 level is still predicted around 1000.
My question is: is it possible at all to brew a beer with such an immence Bicarbonate level?
Wouldn't the hops (which the recipe sets at circa 30 IBU with 4 charges) get unthinkably harsh at such a HCO?
If so, what other means can be employed to raise the pH?
Starting with my untreated tap water of circa 270 ppm Bicarbonate, I'm getting an extremely low mash pH prediction of 3.50 in Brun Water. Further calculations show that upping the pH to 5.5 first with Baking Soda (stopping at going past 60 ppm Na) and then with Pickling Lime would result in insane Bicarbonate level of circa 1640 ppm.
Even if I substitute the Dark 170L Crystal with the Medium 60L, the HCO3 level is still predicted around 1000.
My question is: is it possible at all to brew a beer with such an immence Bicarbonate level?
Wouldn't the hops (which the recipe sets at circa 30 IBU with 4 charges) get unthinkably harsh at such a HCO?
If so, what other means can be employed to raise the pH?














































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)