I have ordered some Gypsum as I want to make my tap water more 'Burtonised' so I can make a nicer IPA.
Now I am a total noob at this, but thought if I bought the gypsum and know what my levels are, Brewers Friend would tell me how much to add, but its not that simple (unless I am doing it wrong!!).
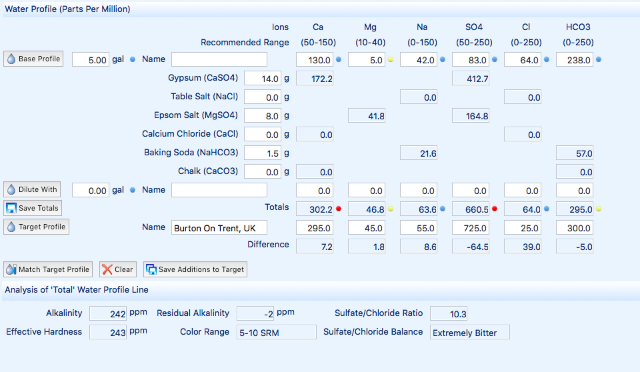
I got these details from my suppliers water report...
Ca+2 Mg+2 Na+ Cl- SO4-2 Alkalinity pH
130 5 42 63 83 238 (HCO3) 8
The report did not give me the Magnesium level, or the PH... So I guessed the MG by taking it from someone else nearby, and PH was 8 by default on Brewers Friend.
The gypsum will add to the Ca and SO right? What kind of levels should I be going for for the IPA? I'm thinking take the Sulphate up to 180 or so?? But the Ca is already quite high, so would this have a negative affect?
Now I am a total noob at this, but thought if I bought the gypsum and know what my levels are, Brewers Friend would tell me how much to add, but its not that simple (unless I am doing it wrong!!).
I got these details from my suppliers water report...
Ca+2 Mg+2 Na+ Cl- SO4-2 Alkalinity pH
130 5 42 63 83 238 (HCO3) 8
The report did not give me the Magnesium level, or the PH... So I guessed the MG by taking it from someone else nearby, and PH was 8 by default on Brewers Friend.
The gypsum will add to the Ca and SO right? What kind of levels should I be going for for the IPA? I'm thinking take the Sulphate up to 180 or so?? But the Ca is already quite high, so would this have a negative affect?






























![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)




























