I spent ages setting this up as an example before homing-in on the probable suspect, and it wasn't what I'd hoped! With hindsight I should have noted
@Yooper's post:
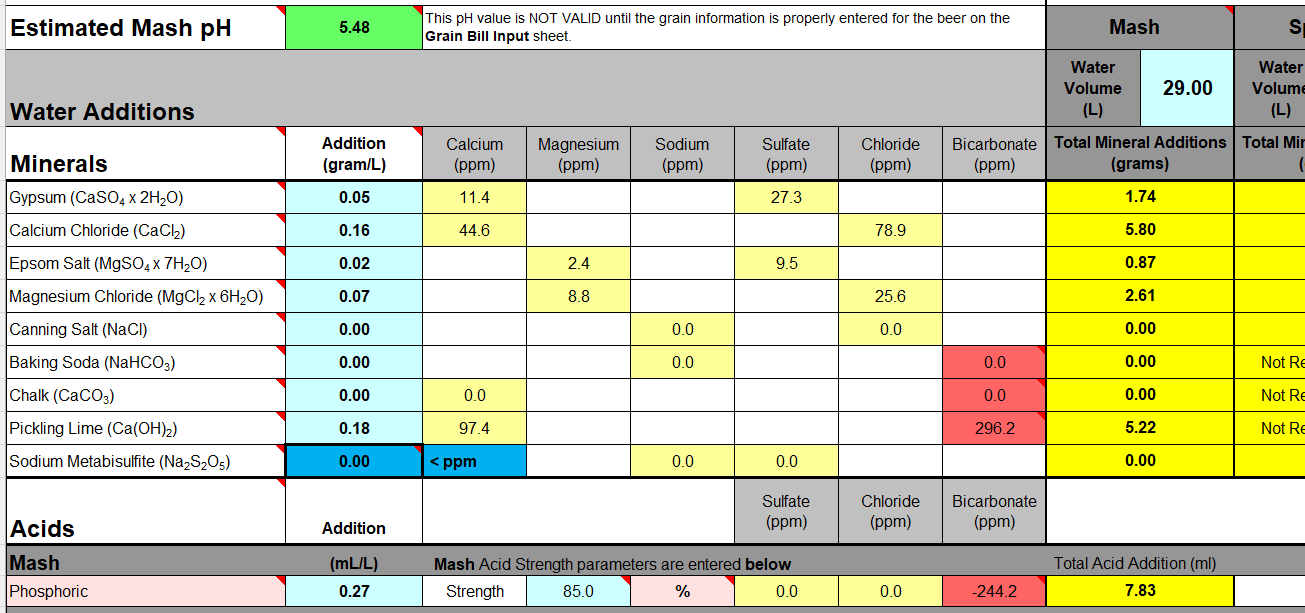
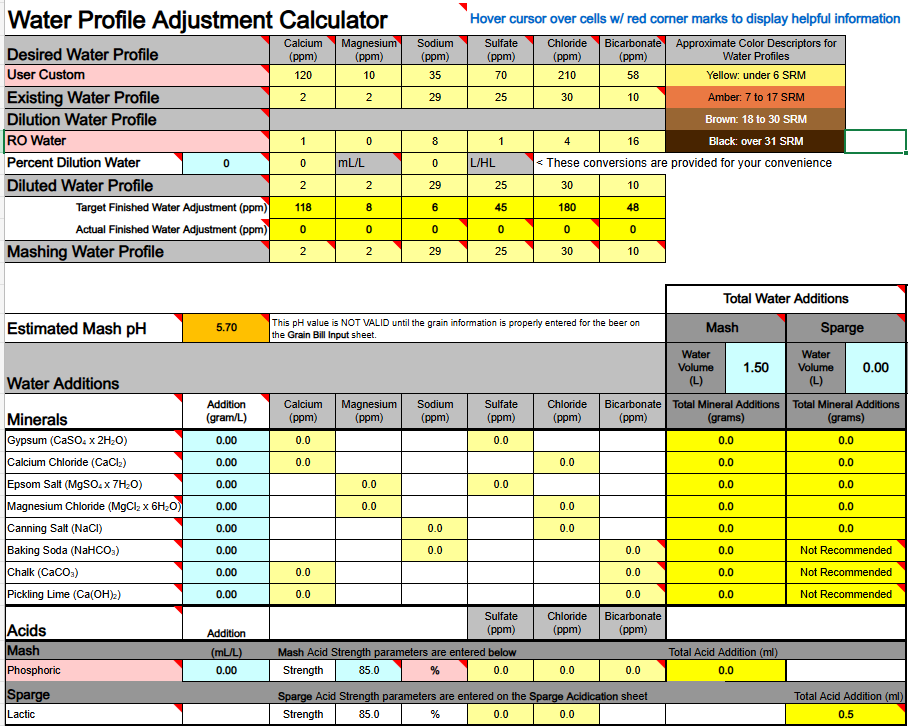
Oddly enough (even odder?) I was achieving this even with acid and alkalinity salts at the same time:
View attachment 866309
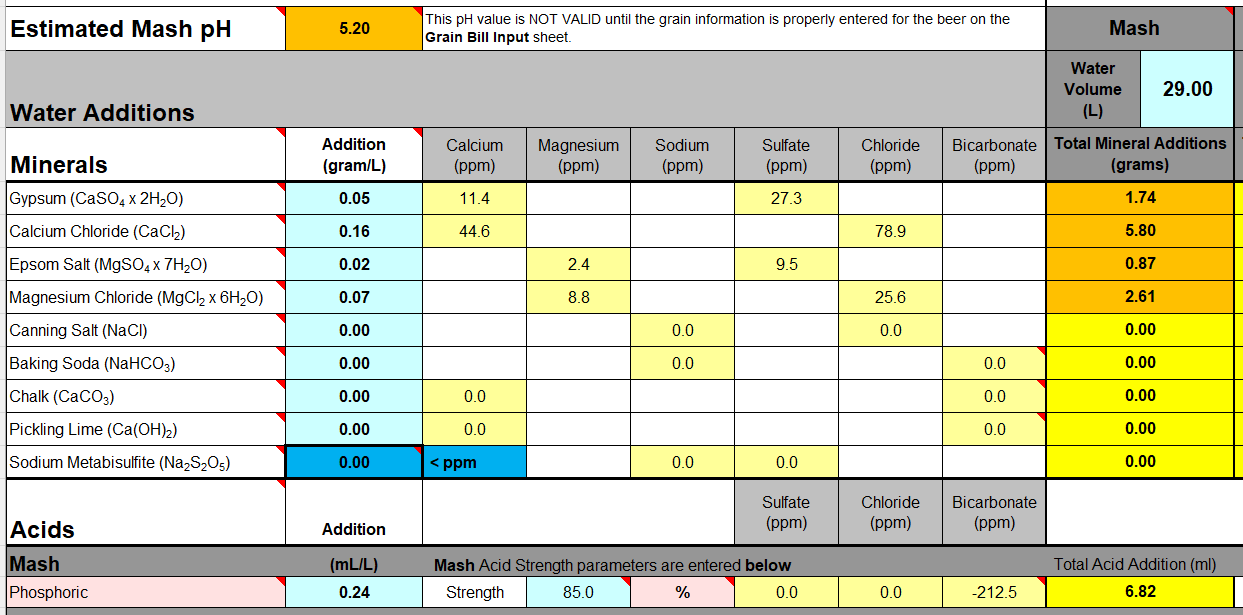
So you can over-ride the acid addition with enough Lime ... but it's not good practice (i.e. pointless?).
Note I'm using the "registered" version, Martin only asks for a token amount to register ... do it! (That might get me another "like" from Martin

). Actually ... I moan like mad about those red blotches (there goes me "like"!

).
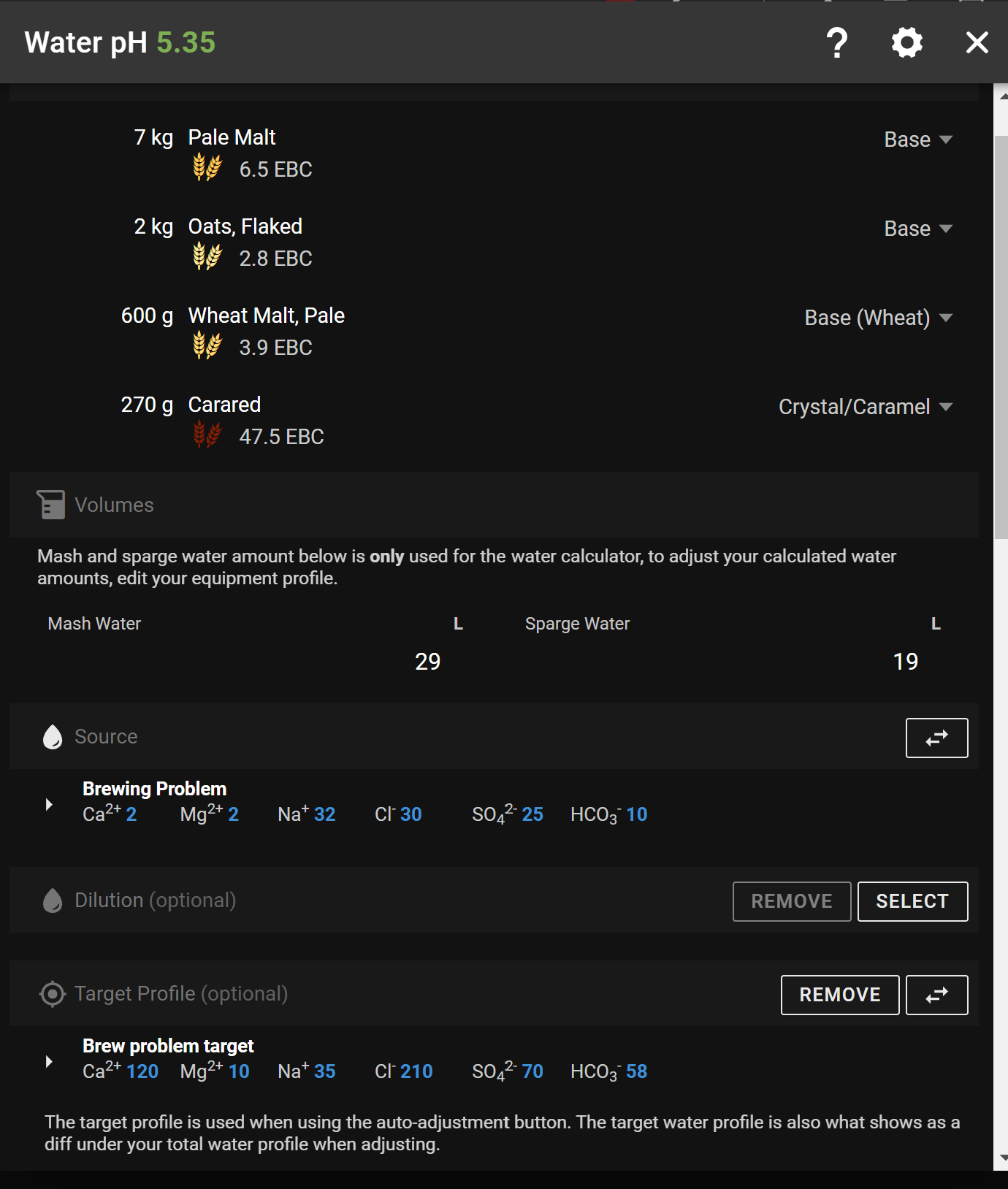
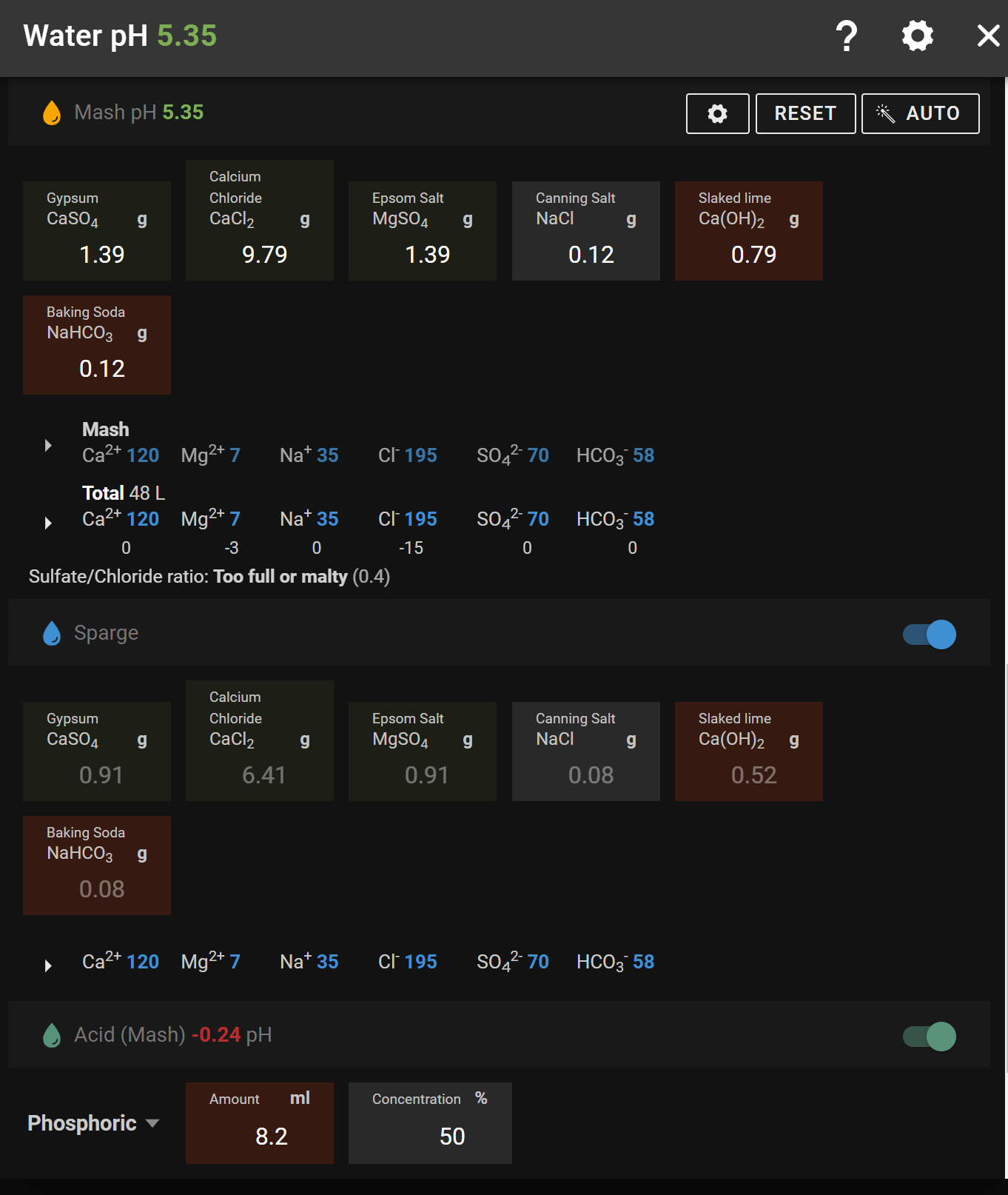
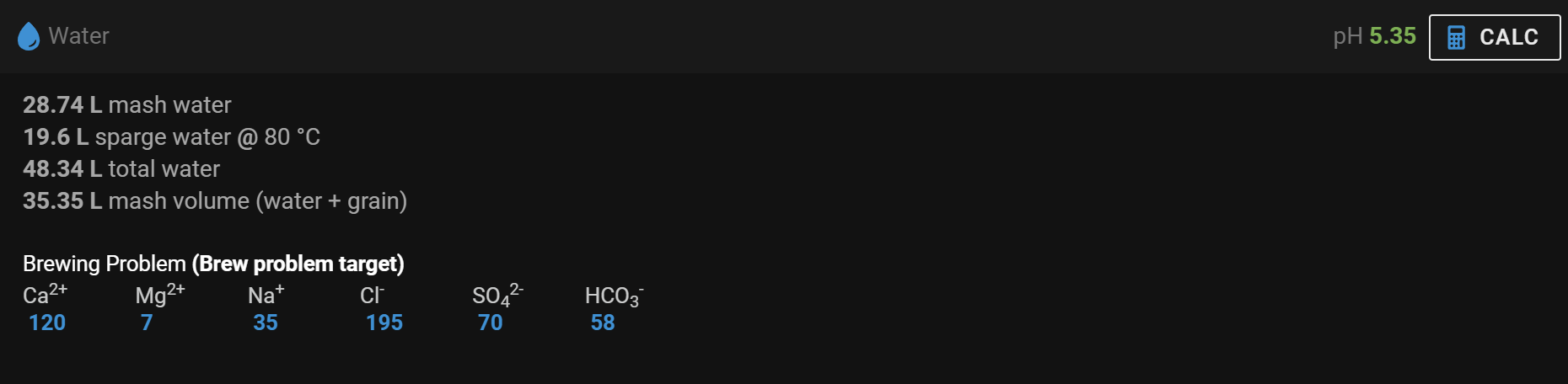
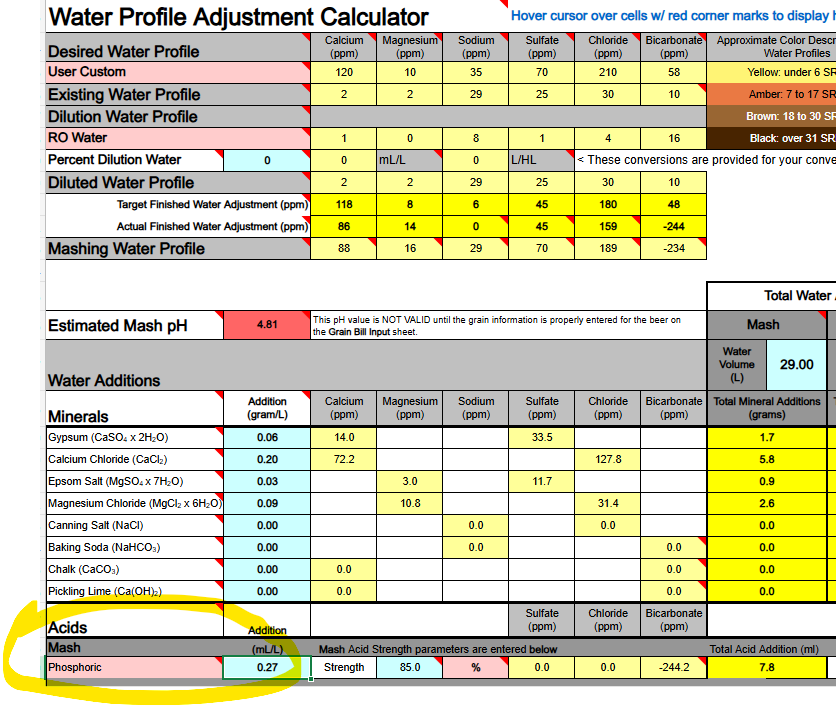
Pay no heed to the "Additions" column in my illustration ... they've been "hacked" 'cos I was only working on the Mash additions.
Also: Bru'n Water is a calculator not a brewing simulator, so none of this need parallel
@sarandos25's
actual problem?
(... Err, but you use Bru'n Water as a brewing simulator all the time ... Shutup you, they don't need to know that ... ).