You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
A Brewing Water Chemistry Primer
- Thread starter Yooper
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
- Status
- Not open for further replies.
All mineral amounts should be halved i.e. where the baseline calls for a tsp. use a half. For places where doubling the baseline is recommended double the new base line (1/2 * 2 = 1 tsp).
Is this meant to be an overall adjustment to the Primer? Or just an adjustment to a particular situation Jiffster posted??
If it's for an overall adjustment, hopefully the original post will be edited accordingly for posterity...
Yes, for the whole primer. I've been saying for quite a while that it should be redone as tastes seem to be for the less minerally now and Yooper has asked me to redo it. It will be the first of my projects when I get up north in a couple of weeks.
Yes, for the whole primer. I've been saying for quite a while that it should be redone as tastes seem to be for the less minerally now and Yooper has asked me to redo it. It will be the first of my projects when I get up north in a couple of weeks.
Wouldn't putting less of the salts bring the pH up? Should there be more of the acid malt added?
Not appreciably and, therefore, no.
Not appreciably and, therefore, no.
How is it not applicable? We add gypsum and sodium chloride to the water and it brings the pH down. If you put less of the salts the pH will be higher.

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid Hose Barb)
Guangshui Weilu You Trading Co., Ltd

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Liquid MFL)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$27.29 ($13.64 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$20.94
$29.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.com

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$172.35
2 Inch Tri Clamp Keg Manifold With Ball Lock Posts, Pressure Gauge, PRV (0-30 PSI) – Homebrew, Fermentation, Kegging System
wuhanshijiayangzhiyimaoyiyouxiangongsi

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$28.98
Five Star - 6022b_ - Star San - 32 Ounce - High Foaming Sanitizer
Great Fermentations of Indiana

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive
appreciableHow is it not applicable?
We add gypsum and sodium chloride to the water and it brings the pH down. If you put less of the salts the pH will be higher.
Not appreciably.
Do some calcs using one of the spreadsheets. How much calcium does it take to lower the pH of the mash by 0.05 pH according to the spreadsheet? Now double that number. Kohlbach's finding was for knockout wort and the effect, therefore, is less for the mash. As a fascinating illustration of not being able to see the forest for the trees I translated Kohlbach's paper and failed to notice that point!
Kohlbach found that each mEq Ca++ released 1/3.5 mEq of protons to knockout. I guess that in the mash it's probably half that. I you wan't to take this into account in your calculation then go ahead and do so but it is not consistent with the concept of the primer.
To be more concrete with an example I happen to have a beer with mostly base malt and some dark caramel in my spreadsheet at the moment. With 1 tsp CaCl2 in 5 gal the sauermalz requirement for mash pH 5.4 was 2.8%. With half that miich (1/2 tsp per 5 gal) it goes up to 3.1%. At the level of accuracy of the Primer that is 3% in either case.
It is a good question but the answer is that it doesn't matter enough to be worrying about at the Primer level.
appreciable
Not appreciably.
Do some calcs using one of the spreadsheets. How much calcium does it take to lower the pH of the mash by 0.05 pH according to the spreadsheet? Now double that number. Kohlbach's finding was for knockout wort and the effect, therefore, is less for the mash. As a fascinating illustration of not being able to see the forest for the trees I translated Kohlbach's paper and failed to notice that point!
Kohlbach found that each mEq Ca++ released 1/3.5 mEq of protons to knockout. I guess that in the mash it's probably half that. I you wan't to take this into account in your calculation then go ahead and do so but it is not consistent with the concept of the primer.
To be more concrete with an example I happen to have a beer with mostly base malt and some dark caramel in my spreadsheet at the moment. With 1 tsp CaCl2 in 5 gal the sauermalz requirement for mash pH 5.4 was 2.8%. With half that miich (1/2 tsp per 5 gal) it goes up to 3.1%. At the level of accuracy of the Primer that is 3% in either case.
It is a good question but the answer is that it doesn't matter enough to be worrying about at the Primer level.
Ah ok, I misunderstood or first response (I thought it was an autocorrect issue). Makes sense!
Is a Centennial Blonde considered a softwater beer?
Trying to determine how to apply the primer to Biermuncher's Centennial Blonde recipe I'm brewing tomorrow using RO water.
It's a light non-hoppy beer. Using calcium chloride and no gypsum would be great.
Jiffster
Well-Known Member
- Joined
- Aug 8, 2015
- Messages
- 806
- Reaction score
- 109
It's a light non-hoppy beer. Using calcium chloride and no gypsum would be great.
1/2 teaspoon calcium per 5 gallon and 3% acid malt, correct?
I've been having a hard time understanding water additives. I use brewersfriend as my software, this will be for a cream ale found here on the site, using 100 ro water with the light and malty profile loaded. Does this seem correct?
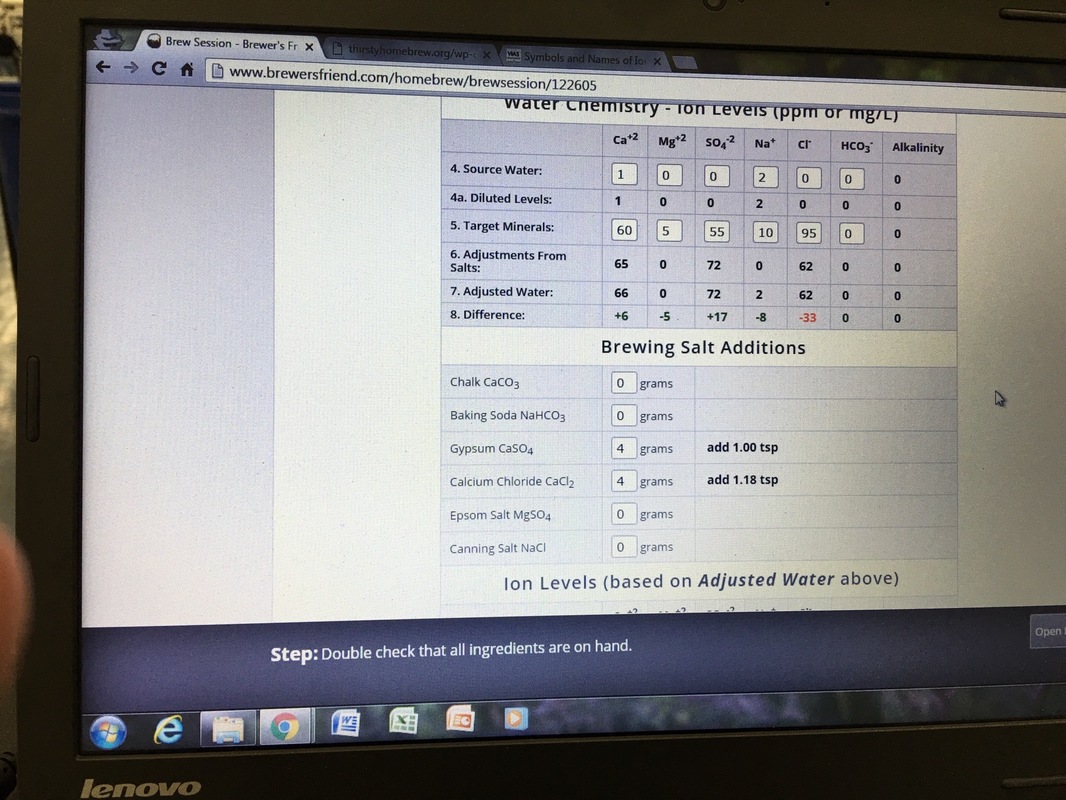

I've been having a hard time understanding water additives. I use brewersfriend as my software, this will be for a cream ale found here on the site, using 100 ro water with the light and malty profile loaded. Does this seem correct?
I'd use less gypsum and more calcium chloride.
brewbama
Well-Known Member
- Joined
- Sep 20, 2013
- Messages
- 3,802
- Reaction score
- 2,829
For British beers: Add .5 tsp gypsum as well as .5 tsp calcium chloride. Is this in addition to the base .5 tsp calcium chloride already in the baseline? Or is this .5 tsp gypsum + .5 tsp calcium chloride total?
I'd use less gypsum and more calcium chloride.
What would be the benefit of doing that? i guess i need to do more reading so I have a better understanding of what those two things change
What would be the benefit of doing that? i guess i need to do more reading so I have a better understanding of what those two things change
Gypsum (sulfate) aids in the accentuation of hops in hoppy beers. Since this is a cream ale, I assume you don't want it to be overly hoppy. Chloride aids in bring out slightly more malt flavors.
Ah ok, thank you very much for clearing that up for me!Gypsum (sulfate) aids in the accentuation of hops in hoppy beers. Since this is a cream ale, I assume you don't want it to be overly hoppy. Chloride aids in bring out slightly more malt flavors.
Gypsum (sulfate) aids in the accentuation of hops in hoppy beers. Since this is a cream ale, I assume you don't want it to be overly hoppy. Chloride aids in bring out slightly more malt flavors.
Sulfate does not accentuate hops. It drys the beer's finish which can allow hopping and bittering to be more prominent in the flavor. If neither of those elements are really present in the recipe, the sulfate won't add them. Some sulfate in beers is helpful in clearing the palate and making the beer more drinkable.
Sulfate does not accentuate hops. It drys the beer's finish which can allow hopping and bittering to be more prominent in the flavor. If neither of those elements are really present in the recipe, the sulfate won't add them. Some sulfate in beers is helpful in clearing the palate and making the beer more drinkable.
How does it dry the beers finish? Allows the yeast to consume more sugar? Or is the drying just a perception?
It is due to the perception in your mouth. It does not alter the wort attenuation.
Sulfate does not accentuate hops. It drys the beer's finish which can allow hopping and bittering to be more prominent in the flavor. If neither of those elements are really present in the recipe, the sulfate won't add them. Some sulfate in beers is helpful in clearing the palate and making the beer more drinkable.
The definition of accentuating is to make more prominent so we are saying the same thing. You said sulfate drys out the beer which makes the hop flavor more prominent. Wouldn't that be the same as sulfate accentuates the hop flavor?
I said sulfate aids in the accentuation of hops meaning adding sulfate to a hopped beer would make the hop flavor more prominent (by drying out the beer). For sulfate to be able to aid in the accentuation of hops, hops already need to be in the beer. If hops aren't in the beer, there is nothing for sulfate to aid. Maybe my wording should have been clearer.
I probably should have said, adding gypsum (sulfate) to a hopped beer will help bring out the hop flavor.
SnowRaven
Well-Known Member
- Joined
- Jan 6, 2014
- Messages
- 128
- Reaction score
- 9
Using calcium chloride and no gypsum would be great.
Any recommendation on the Atomic Amber (Home Brew All Stars Recipe)
Northwestern Style Amber ~6.7% 44 IBU ?
Water is 100% RO DI - 8 ppm TDS
has 4 oz of late hops in it (bittered with Magnum 21 g) 15.8 lbs of grain
Go balanced .5 Gypsum .5 CaCl
or go
1 tsp CaCl ?
any Epsom?
Fermentables
Amount Fermentable PPG °L Bill %
13.5 lb American - Pale 2-Row 37 1.8 85.2%
10 oz American - Caramel / Crystal 40L 34 40 3.9%
5 oz American - Caramel / Crystal 90L 33 90 2%
10 oz American - Victory 34 28 3.9%
4.25 oz United Kingdom - Chocolate 34 425 1.7%
5 oz German - CaraMunich I 34 39 2%
3.25 oz German - Acidulated Malt 27 3.4 1.3%
15.84 lb Total
Hops
Amount Variety Type AA Use Time IBU
21 g Magnum Pellet 15 Boil 60 min 28.69
1 oz Cascade Pellet 7 Boil 10 min 6.55
1 oz Centennial Pellet 10 Boil 10 min 9.36
1 oz Cascade Pellet 7 Boil 0 min
1 oz Centennial Pellet 10 Boil 0 min
Dcpcooks
Well-Known Member
Ok! I went all in. Got an RO system from BuckeyeHydro. Based on the TDS meter my solids are at 1 down from 158. (Chicago area water close to the lake)
Brewing a RIS tomorrow. Based on the recco I'm adding 6g calcium CaCl for 30 gallons of water. Treating everything the same. I'll update as soon as I get it finished and 4-6 months (barrel aged).
Thanks to yooper and AJ for making this mind bender of a chemical nightmare easy for a poor old chef who failed Chem II.
Brewing a RIS tomorrow. Based on the recco I'm adding 6g calcium CaCl for 30 gallons of water. Treating everything the same. I'll update as soon as I get it finished and 4-6 months (barrel aged).
Thanks to yooper and AJ for making this mind bender of a chemical nightmare easy for a poor old chef who failed Chem II.
MagicMatt
Brewmathemagician
Based on the recco I'm adding 6g calcium CaCl for 30 gallons of water.
Just a heads up, but I believe you mean "6 tsp", not "6g". The Primer states 1 tsp / 5 gal. For 30 gallons, this would equal about 20g CaCl (assuming roughly 3.5g/tsp).
And I think the the recommendation is half of the stated primer so it's 10.2g. That's at 3.4g/tsp. Confusing enough?
Dcpcooks
Well-Known Member
Just a heads up, but I believe you mean "6 tsp", not "6g". The Primer states 1 tsp / 5 gal. For 30 gallons, this would equal about 20g CaCl (assuming roughly 3.5g/tsp).
Yes tsp not g's. Typo. Thank you
I acknowledge this is probably a completely stupid question but my home brewer paranoia is kicking in. I use RO water I buy from a water store in sealed 5 gallons container. Fill your own jugs kind of place. For various reasons I haven't been able to brew when I expected to so by the time I use this water it will be about a month old since I got it. So the stupid question is, does it go stale, "bad"... after sitting around? If it somehow isn't as good as fresh water I'd rather just buy more when I will finally end up brewing. Thanks!
Why did the British Navy carry small beer on board its vessels?
Yes, water can go bad. Life is amazingly tennacious. Give it a carbon source (CO2 in the air), energy (heat) and a seed (spore) and it will develop in even pure water. Thus water stored in barrels on board ship went bad and had to be replaced by beer (one of the few good things about being in the British Navy from what I gather). At the same time, I have an RO system at home with a pressure tank and atmospheric tank. I am away 5 - 6 mos every summer and when I return in the fall the water in the pressure tank is fine. But I don't trust the water exposed to the air in the atmospheric tank and drain that when I go away.
I'd say that water that tastes and smells OK and doesn't have slimy things growing in it is fine for use in brewing.
Yes, water can go bad. Life is amazingly tennacious. Give it a carbon source (CO2 in the air), energy (heat) and a seed (spore) and it will develop in even pure water. Thus water stored in barrels on board ship went bad and had to be replaced by beer (one of the few good things about being in the British Navy from what I gather). At the same time, I have an RO system at home with a pressure tank and atmospheric tank. I am away 5 - 6 mos every summer and when I return in the fall the water in the pressure tank is fine. But I don't trust the water exposed to the air in the atmospheric tank and drain that when I go away.
I'd say that water that tastes and smells OK and doesn't have slimy things growing in it is fine for use in brewing.
Why did the British Navy carry small beer on board its vessels?
Yes, water can go bad. Life is amazingly tennacious. Give it a carbon source (CO2 in the air), energy (heat) and a seed (spore) and it will develop in even pure water. Thus water stored in barrels on board ship went bad and had to be replaced by beer (one of the few good things about being in the British Navy from what I gather). At the same time, I have an RO system at home with a pressure tank and atmospheric tank. I am away 5 - 6 mos every summer and when I return in the fall the water in the pressure tank is fine. But I don't trust the water exposed to the air in the atmospheric tank and drain that when I go away.
I'd say that water that tastes and smells OK and doesn't have slimy things growing in it is fine for use in brewing.
Thanks I appreciate it!
I acknowledge this is probably a completely stupid question but my home brewer paranoia is kicking in. I use RO water I buy from a water store in sealed 5 gallons container. Fill your own jugs kind of place. For various reasons I haven't been able to brew when I expected to so by the time I use this water it will be about a month old since I got it. So the stupid question is, does it go stale, "bad"... after sitting around? If it somehow isn't as good as fresh water I'd rather just buy more when I will finally end up brewing. Thanks!
I think there are 3 things that could happen: algae/mold/bacteria growth: Might impart flavor, not that big a deal preboil.
Plastic/BPA over time can impart the water for a plastic flavor.
Depending on the seal, the pH could change as some of the water forms carbonic acid from the atmosphere.
All in all: keep a clean bottle before filling, store in a cool dark place, and don't worry about it
- Status
- Not open for further replies.
Similar threads
- Replies
- 2
- Views
- 620
- Replies
- 19
- Views
- 2K
- Replies
- 3
- Views
- 606






























![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)















