That system had a condenser temperature in the 80's. It would never, normally operated, reach 30 °F. But you could make it do so by removing refrigerant (antoher way to starve the evaporator). It would be terribly inefficient (so that you would have to lighten the load) but I don't think anyone cares about that here. It would bother me to operate it well outside its design parameters but if the attitude is 'run it until it goes TU' then why not?
..the second law of thermodynamics tells us that no more heat can flow from the glycol to the coolant.
You are going to think me terribly pedantic but it is the Zeroth law that is at play here. The Second Law implies that it is harder (takes more work) to pump a joule of heat from 20 ° to 70 ° than from 40 ° to 70 °.
As the temperature of the glycol continues to decrease in temperature the coolant will superheat to a lesser extent.
As you starve the evaporator, which you will have to do if you can't get the condenser cooled enough, the superheat will actually go up. But that is a good thing. As superheat gets low you start to run the risk of slugging the compressor. This is why it is, IMO, important that you get a gauge set and see what is actually going on in your system(s?). It will cost you about as much as a decent pH meter ($150 or so). All this speculation on your part and mine is really meaningless when stacked up against real data.
When (if it can) the glycol solution reaches the setpoint of 30°F the low side pressure will no longer be the 119psig design and the discharge will no longer be superheated without violating the second law of thermo.
Have a look at the heat pump data again. There we have evaporator out gas at about 30 °F which could, with a good heat exchanger, get us close to 30 °F on the liquid side. In the case of the actual heat pump the reservoir is at 50 °F and were getting that by being able to cool the condenser output liquid to around 60 °F. Again, I think you want the zeroth law. The second law speaks to the efficiency of a system.
The liquid-liquid graph you show at the bottom of your last post is a differently designed system than the air-air we were previously discussing, at (what I assume without axis labels) 80psig subcooling the saturation temp is right below 0°F (assuming units again) and the ~15°F superheat puts the condenser at a lower temperature than a bath set at 30°F.
Yes, sorry about not labeling the axes. I do enough of these that I don't have to and I meant to put the labels on this one but forgot. As you probably figured out the left axis is pressures (gauge) and the right temperatures in °F.
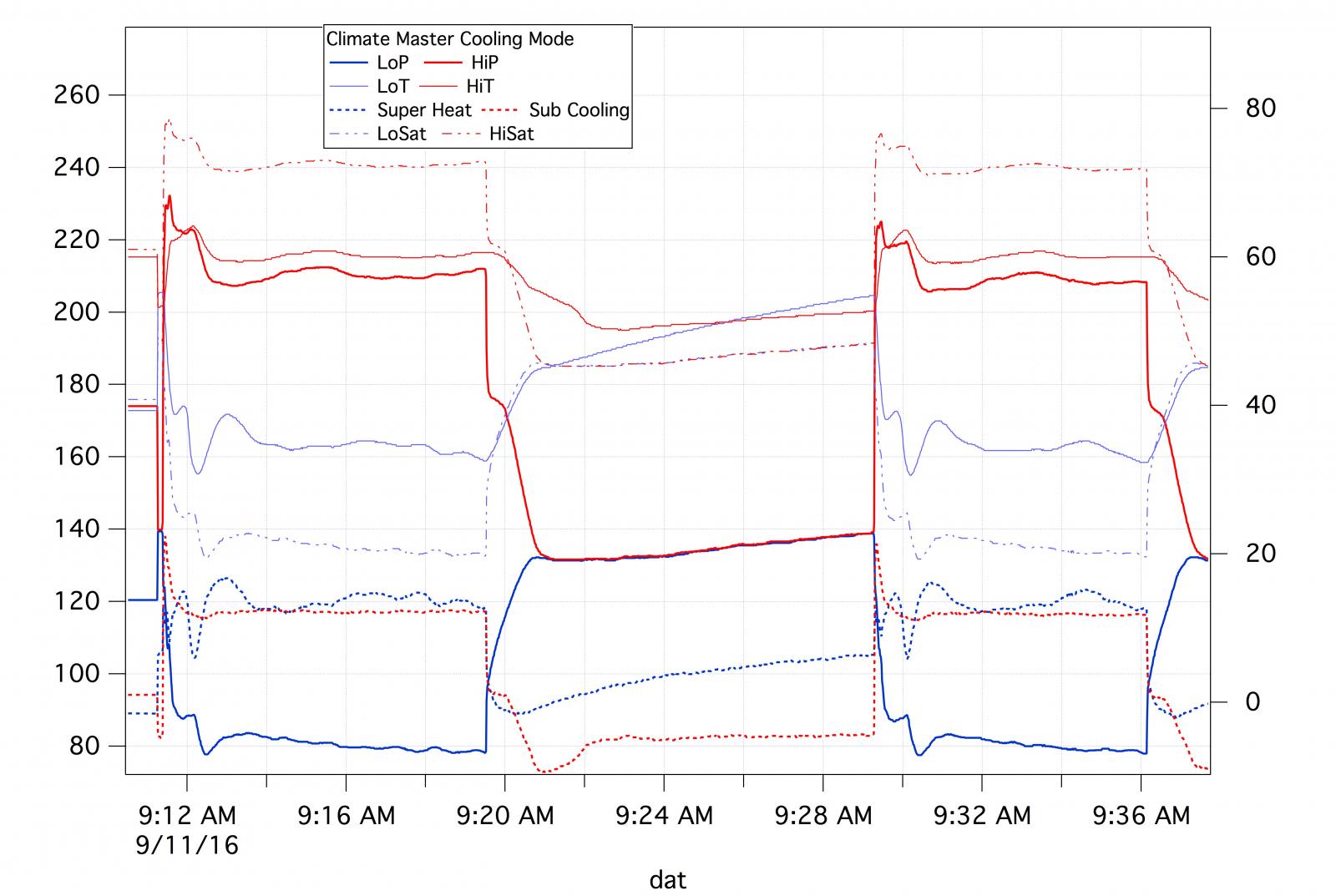
For starters, the unit is cycling. There are two on periods and one off which is clearly demarcated by the hi and low pressures coming together.
No one would design a system to operate at 80° subcooling. That is indicated on the right hand scale and is about 10 ° as is the superheat. The low side boiling point is about 20 °F.
Setting the temperature lower than the superheat would cause the same evaporator = bath condition as the above A/C Example.
I'm not sure what you mean by this. A super heat of 10 - 12 is pretty common and you could have, in a walk in freezer, for example, a low side temperature of less than this. To reiterate, the superheat is about 12, the low side temperature about 32 and the low side saturation temperature about 20.
Yep, and this is where we get to what I think we are disagreeing/discussing
It is, for the most part, discussion. You have/had some misconceptions but they are, I think, pretty much cleared up.
Yes a properly designed glycol cooler looks like it would have the same 3:1 compression ratio of a A/C;
In the larger central systems the scroll compressors are now very popular because of their efficiency, low sound/vibration levels, longevity and resistance to slugging. But they don't seem to provide in practice more than about 2.4:1 compression ratios.
...however both low and high side design pressures are lower than the A/C.
The design pressures depend on the application. A liquid/liquid refrigeration machine is not designed to cool air. It is designed to cool liquid. Now the cooled liquid may be used to cool air or beer or whatever.
As such the system can cool to a lower temperature, but requires a lower condenser side temp.
You can make an air to air system cool to a tempreature as low as a glycol system. The designs are essentially the same (an oboe and a bassoon are really the same thing: double reeded wood winds) but the details differ. To go to lower temperature you need a colder condenser or a better compressor or both.
Seeing this math laid out makes me realize that someone using an A/C system or a purpose built glycol system in an unconditioned area may have issues with low temperature operation.
With compressors of limited capability you must do (if you insist on having a 20 ° reservoir) what a cryogenic designer does: use a multi stage system in which the condenser of the low temperature loop is cooled by the evaporator in the high temperature loop. In this case, of course, the high temperature 'loop' is your room or central air conditioner but the principle is the same.
It also makes me realize that the efficiency loss of my system at low set temperatures (were I keep it) is much greater than I was estimating (I knew I was shifting the "curve" but didn't realize that I got rid of all the superheat and was operating so far below the design Low pressure point).
This is where the Second Law comes in. The COP for a refrigerator is Tc/(Th-Tc) thus the closer the evaporator and condenser temperatures can be the higher the efficiency.
Nope, no HVAC tools. I just bent the pliable copper lines to fit where I wanted without evacuating the system.
Amazing! That's pretty cooperative copper, I'd say. I still think a gauge set would be an awfully good investment. My speculations taken from PE charts and tables are fine for elucidating the principles involved but there is nothing like solid numbers.



 (sorry, most of your posts that I see are on chemistry and you obviously have a great handle on that
(sorry, most of your posts that I see are on chemistry and you obviously have a great handle on that 






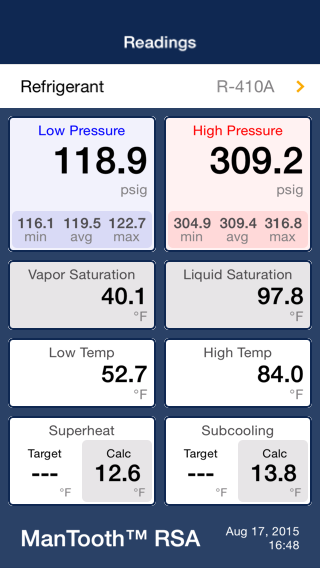


 But I would guess at 15°F he is not changing the vapor-compression cycle directly but simply operating off design enough that he has dropped BTU rating the same way I am.
But I would guess at 15°F he is not changing the vapor-compression cycle directly but simply operating off design enough that he has dropped BTU rating the same way I am. from original conversation.
from original conversation.