So, I’m trying my first brew starting with RO water. I’m new to water calculations and just want to try and keep it simple. I’ve read some info on the subject but, by no means do I understand the in’s and out’s. I’m making an American pale ale.
Recipe:
4lb 5oz pale malt (2 row)
4lb 5oz Pilsner (2 row)
1lb aromatic malt
1lb cara-pils/dextrin malt
1lb crystal-60 malt
1oz Columbus at 60 min.
.25oz Amarillo at 30 min.
.25oz cascade at 25 min.
.25oz Amarillo at 20 min.
.25oz cascade at 15 min.
.25oz Amarillo at 10 min.
.25oz cascade at 5 min.
.25oz Amarillo at flameout.
.25oz cascade at flameout.
California ale yeast.
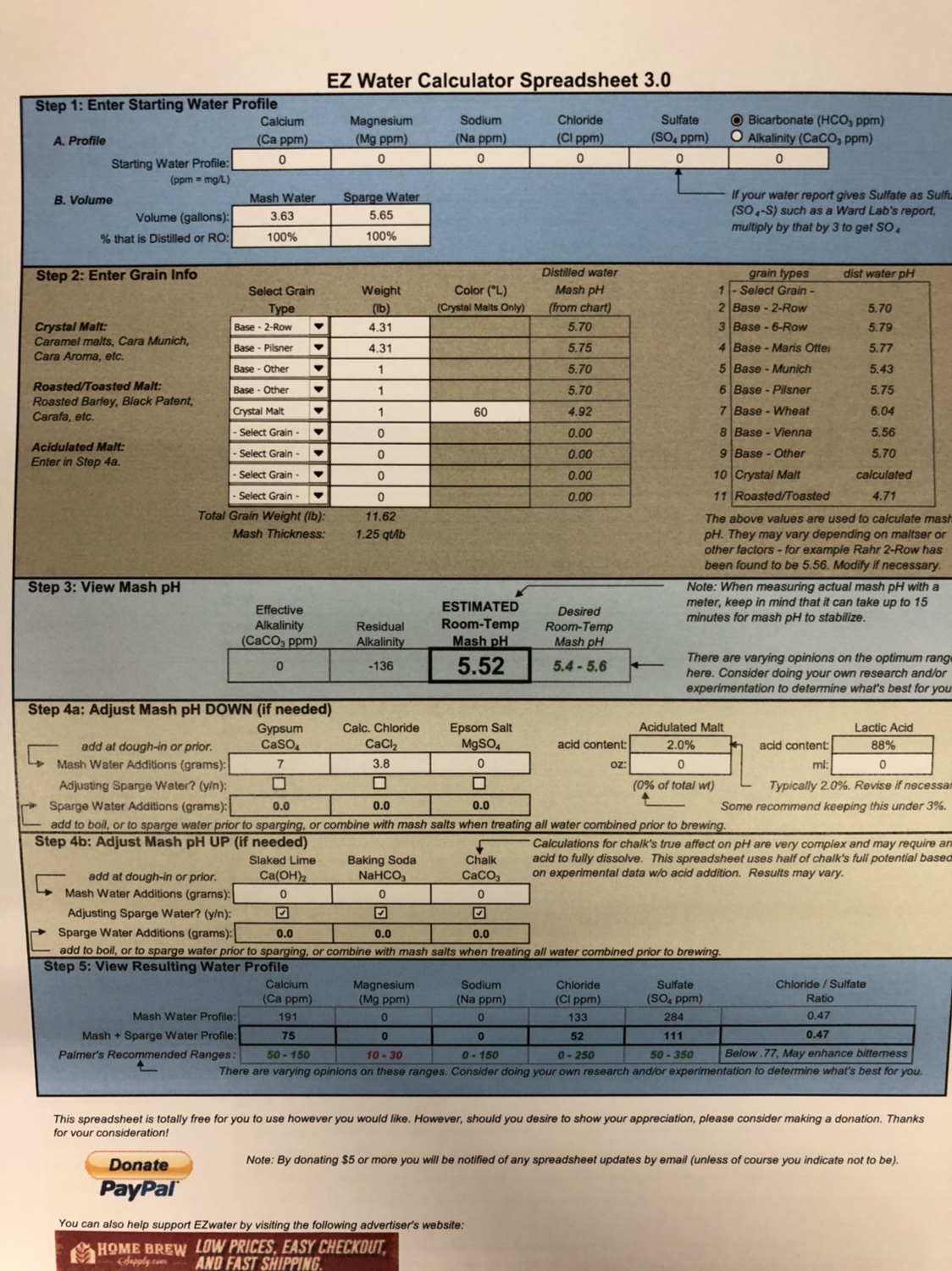
Here is what I have so far for the water profile. I bought calcium chloride and gypsum at my brew shop. From reading what I’ve read on the subject, this is what I came up with. Any suggestions would be appreciated....or if I’m way off, a little help!
I’m also not sure about any acid additions for mash pH but EZ water states that the estimated pH will be 5.52. I did not buy any acid and do not have a meter. Can I accurately go off this? Thanks for the help!
Recipe:
4lb 5oz pale malt (2 row)
4lb 5oz Pilsner (2 row)
1lb aromatic malt
1lb cara-pils/dextrin malt
1lb crystal-60 malt
1oz Columbus at 60 min.
.25oz Amarillo at 30 min.
.25oz cascade at 25 min.
.25oz Amarillo at 20 min.
.25oz cascade at 15 min.
.25oz Amarillo at 10 min.
.25oz cascade at 5 min.
.25oz Amarillo at flameout.
.25oz cascade at flameout.
California ale yeast.
Here is what I have so far for the water profile. I bought calcium chloride and gypsum at my brew shop. From reading what I’ve read on the subject, this is what I came up with. Any suggestions would be appreciated....or if I’m way off, a little help!
I’m also not sure about any acid additions for mash pH but EZ water states that the estimated pH will be 5.52. I did not buy any acid and do not have a meter. Can I accurately go off this? Thanks for the help!



