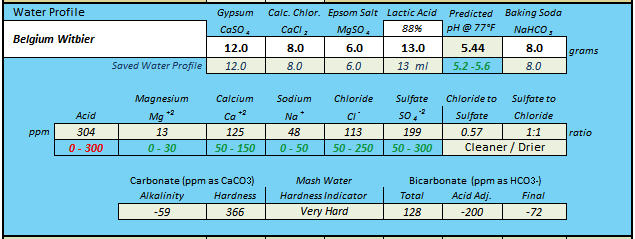
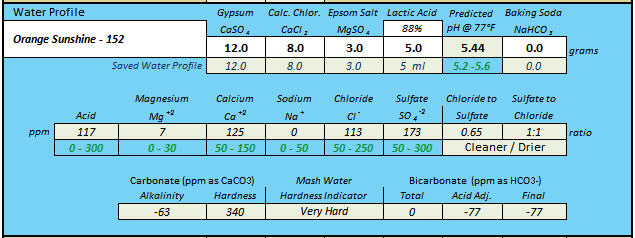
Stand
Well-Known Member
I'm new to water chemistry, and I'm trying to use the calculators I found online to figure this out. I read the thread AJ posted here, and it seems to be pushing me in the opposite direction of everything else I'm reading online.
I have a Ward Labs profile, and I've got all the chemicals to make an adjustment, but this is the first time I'm really stepping into this.
Right now my water profile is as follows:
pH: 7.3
Ca: 9
Mg: 4
Na: 15
SO4: 21
CL: 15
HCO3: 21
I found this thread with the profile provided by mabrungard: https://www.homebrewersassociation.org/forum/index.php?topic=5341.0
Ca 85 ppm
Mg 4 ppm
Na 12 ppm
SO4 55 ppm
Cl 19 ppm
HCO3 200 ppm
I see what he's saying about the problems dissolving chalk, but I also found an article about using CO2 (I keg, so this is possible).
I'm using ezwatercalculator.com spreadsheet to figure out my final numbers, and I can get them where I want them (except chalk seems to be the answer to getting to that profile), and the residual alkalinity drops into negative territory.
Beersmith has me add: .3g Epsom, .2g Baking Soda, .8g Chalk per gallon to reach that profile.
Now I see people screaming never to add alkalinity to mash?
I don't care about matching historical profiles, I just want to know what is essential to brewing a good stout.
The more I read the more confused I get. Can anyone help me with this?
I have a Ward Labs profile, and I've got all the chemicals to make an adjustment, but this is the first time I'm really stepping into this.
Right now my water profile is as follows:
pH: 7.3
Ca: 9
Mg: 4
Na: 15
SO4: 21
CL: 15
HCO3: 21
I found this thread with the profile provided by mabrungard: https://www.homebrewersassociation.org/forum/index.php?topic=5341.0
Ca 85 ppm
Mg 4 ppm
Na 12 ppm
SO4 55 ppm
Cl 19 ppm
HCO3 200 ppm
I see what he's saying about the problems dissolving chalk, but I also found an article about using CO2 (I keg, so this is possible).
I'm using ezwatercalculator.com spreadsheet to figure out my final numbers, and I can get them where I want them (except chalk seems to be the answer to getting to that profile), and the residual alkalinity drops into negative territory.
Beersmith has me add: .3g Epsom, .2g Baking Soda, .8g Chalk per gallon to reach that profile.
Now I see people screaming never to add alkalinity to mash?
I don't care about matching historical profiles, I just want to know what is essential to brewing a good stout.
The more I read the more confused I get. Can anyone help me with this?