BryskeBrynolf
Beereft of all sense
- Joined
- Dec 10, 2016
- Messages
- 12
- Reaction score
- 7
Howdy!
My first attempt at pressure fermentation. Got myself a Fermzilla 30L All Rounder with a spunding valve. Brewed a German(-ish) lager in the usual way. Pitched 2 packs of 34/70 at 10 °C and let it ride with no pressure for the first 3 days. Set the spunding valve at 0.8 bar on day 4 and observed the pressure rise to the desired level over a few hours. Fermentation chugged along as predicted, pressure constant at 0.8 bar. Started ramping up the temp by 1 °C per day. On day 6 fermentation suddenly stopped dead and the pressure started dropping (as expected I guess, since CO2 production ceased).
I had expected the fermentation to slowly subside towards some FG as it normally does, but here, it seems the yeasties were suddenly all obliterated. Does anyone with (or without) experience of pressure fermentation have any idea what happened?
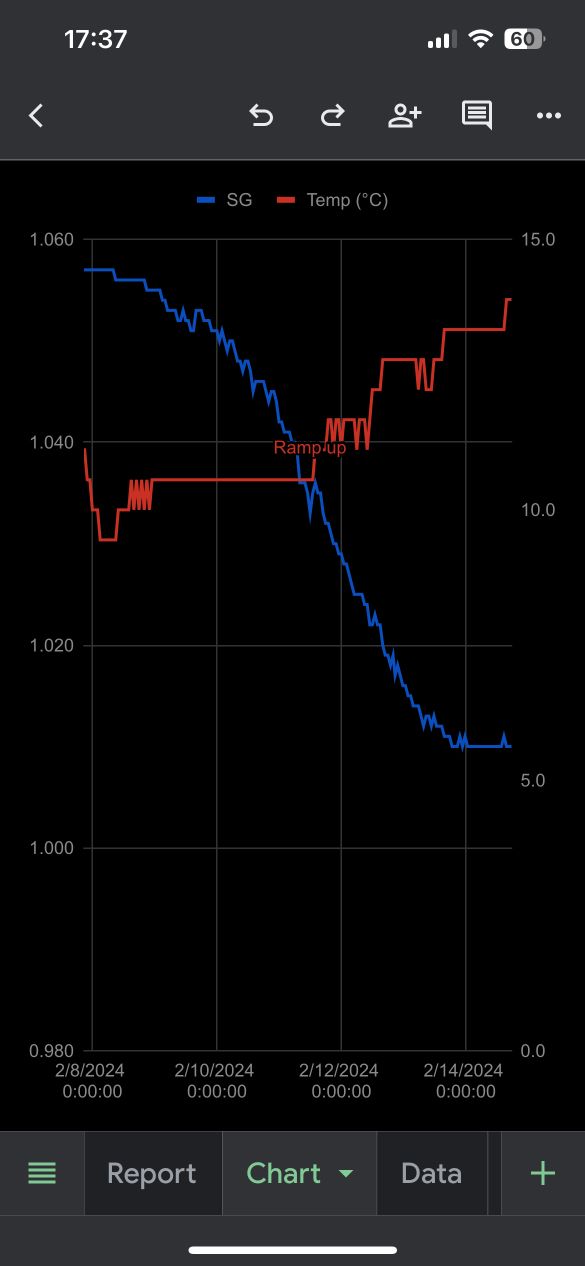
My first attempt at pressure fermentation. Got myself a Fermzilla 30L All Rounder with a spunding valve. Brewed a German(-ish) lager in the usual way. Pitched 2 packs of 34/70 at 10 °C and let it ride with no pressure for the first 3 days. Set the spunding valve at 0.8 bar on day 4 and observed the pressure rise to the desired level over a few hours. Fermentation chugged along as predicted, pressure constant at 0.8 bar. Started ramping up the temp by 1 °C per day. On day 6 fermentation suddenly stopped dead and the pressure started dropping (as expected I guess, since CO2 production ceased).
I had expected the fermentation to slowly subside towards some FG as it normally does, but here, it seems the yeasties were suddenly all obliterated. Does anyone with (or without) experience of pressure fermentation have any idea what happened?

Last edited: