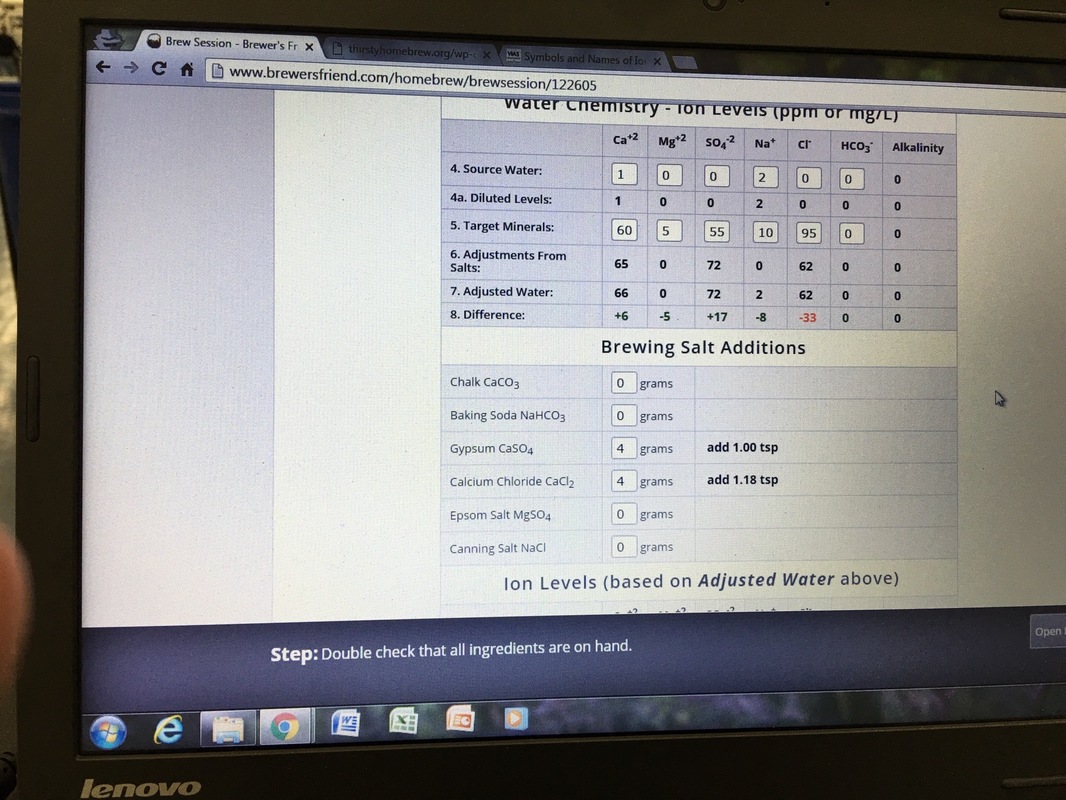
Water chemistry has many old holdovers. One is referencing ions to "as CaCO3" allowing easy calculations. A value for HCO3 of 50 (as CaCO3) is the same as a value of 61.
It actually makes calculations harder because it only makes them easier when calcium carbonate was dissolved by CO2 and that's usually not the case. Anyway, "as CaCO3" is applied to magnesium and calcium hardness and to alkalinity - not bicarbonate in general but popular spreadsheets often assume bicarbonate and alkalinity to be the same thing which they aren't except in a certain range of pH. I wanted to be sure what you meant (and I still am not).
I have several LaMotte test kits. One measures Na, but provides intermediate measurements yielding Total Alkalinity, SO4 combined with CL,
Actually it is measuring the totality of the cations which, as they must be equal to the anions then call the totality of the sulfate and chloride which they are assuming is all the anions but there can be appreciable nitrate and some phosphate in there too. That wouldn't throw off the sodium reading because, as I said, they are going to subtract magnesium and calcium from the total cations and assume the only think left is sodium. The sodium reading will, thus, be off if potassium, iron or manganese are present of which only potassium is likely to make an appreciable difference. Strontium (of which there won't be much if any) will be picked up as 'hardness' and should not make much difference.
and Ca combined with Mg. An additional kit measures Ca, allowing me to disentangle Mg. A third kit measures Cl, allowing disentanglement of SO4. The kits like to speak in "as CaCO3" terms. Thus my use.
Yes. I see that. Sulfate "as CaCO3" is technically OK but just leads to so much confusion. So much clearer if they used mEq/L.
The kits use titration and color change indicators. Using a hypodermic needle to significantly reduce added "droplet" size my accuracy is limited only by my ability to say the color changed the correct amount. Adding a few droplets more confirms the color change and bounds the resulting value.
Lamotte also makes a kit for brewers in which chloride, sulfate, alkalinity and sulfate are measured ant the sodium assumed to be responsible for any charge imbalance (what it necessary to zero the sum of hardness - alkalinity - chloride - sulfate. In the test you describe it is assumed responsible for the cation charge not accounted for by hardness. This gets the sulfate (as read by the BaCl2 Secchi disk test of the brewer's kit) out of the equation and has got to give a much more accurate sodium reading. Pretty clever, actually.
I don't know why SO4 is as high as it is. It may be do to the subtraction of intermediate numbers or the water collection system.
As I mentioned above 'B' is actually the sum of chloride, sulfate, nitrate, nitrite, biphosphate, fluoride so what you are calling 'sulfate' is actually the sum of sulfate, nitrate, nitrite, biphosphate, and fluoride.
I like the idea of walking the minerals in slowly, like 100 ppm after the fact, so add only 50ppm in the mash/sparge.
I see your point about the anion. Typically my water has HCO3 of 61.
I am taking that to mean that your water's alkalinity is 1 mEq/L (50 ppm as CaCO3).
Via titration with 0.1N Sulfuric Acid I can develop a curve mapping pH to remaining HCO3 (down to ~0).
I then add phosphoric or lactic acid (dependent on beer style) to mash and sparge water to hit the pH for the desired HCO3 I wish to leave. Typically for phosphoric (10%) it may be 10ml and lactic (88%) 2ml per 5 gals.
See
https://www.homebrewtalk.com/showthread.php?t=473408
for formulas you can use to calculate the amount of carbo present in a liter, of your water, its titration curve and, thus, exactly how much acid it takes to get a volume of your water to a given pH. There is never any need to consider pH below mash pH as you wont ever be going to such a pH.