Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
After procrastinating for 3 years I finally had my abysmal well water analyzed. Here it is for your amusement. SO4 is actually 186 ppm. Believe it or not, I actually think a number of my recipes can use this water at a blend ratio of 25% well and 75% RO. I can't go higher than 25% and keep iron at the generally accepted maximum limit of 0.1 ppm. Not that I would want to go higher...
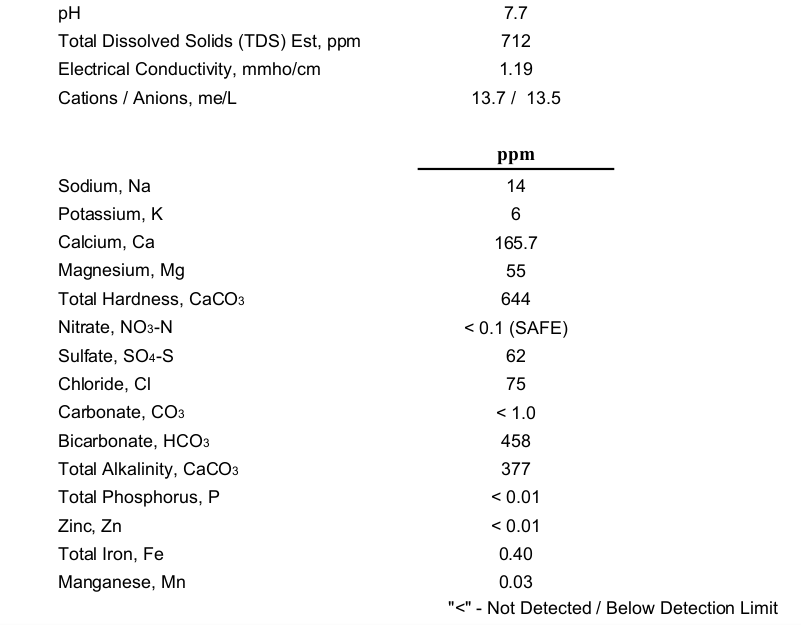
It's nearly as bad as I had originally surmised via using a GH/KH test kit on it, but not quite. I was hoping it would have had a smidge of zinc in it, given everything else it has in abundance.

It's nearly as bad as I had originally surmised via using a GH/KH test kit on it, but not quite. I was hoping it would have had a smidge of zinc in it, given everything else it has in abundance.




















![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)




































