jklett
Active Member
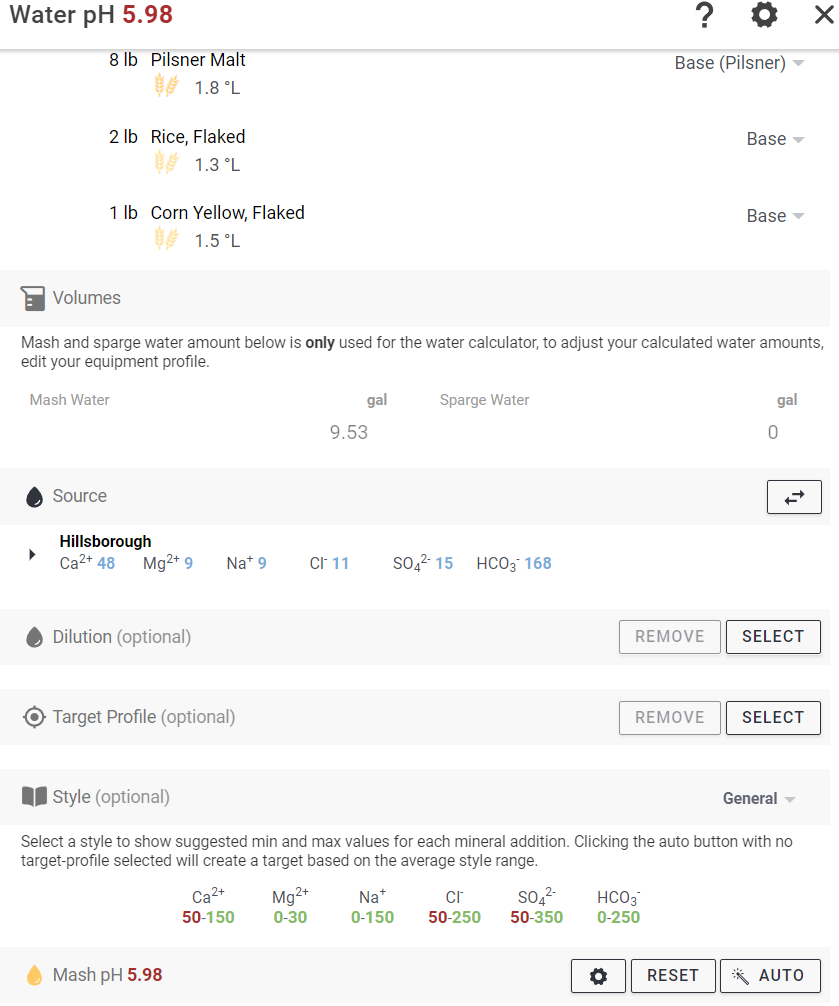
I just got back my water profile. It is a bit Greek to me as of now but hopefully I can figure out what to do with it shortly.
PH = 8.0
TDS = 245 ppm
Electrical conductivity 0.41
Cations / Anions, me/L = 3.5/3.7
Na = 9
K = <1
Ca = 48.4
Mg = 9
CaCO3 = 158
NO3-N = 4.4
SO4-S = 5
Cl = 11
CO3 = <1.0
HCO3 = 168
CaCO3 = 139
P = 0.10
Fe = <1.0
If anybody has any feedback as to if this is a good starting point or not and what direction I should take based on what I have to work with, I'd be very interested in any advice. The beer I've been brewing with it has been good but I am always looking to make it better.
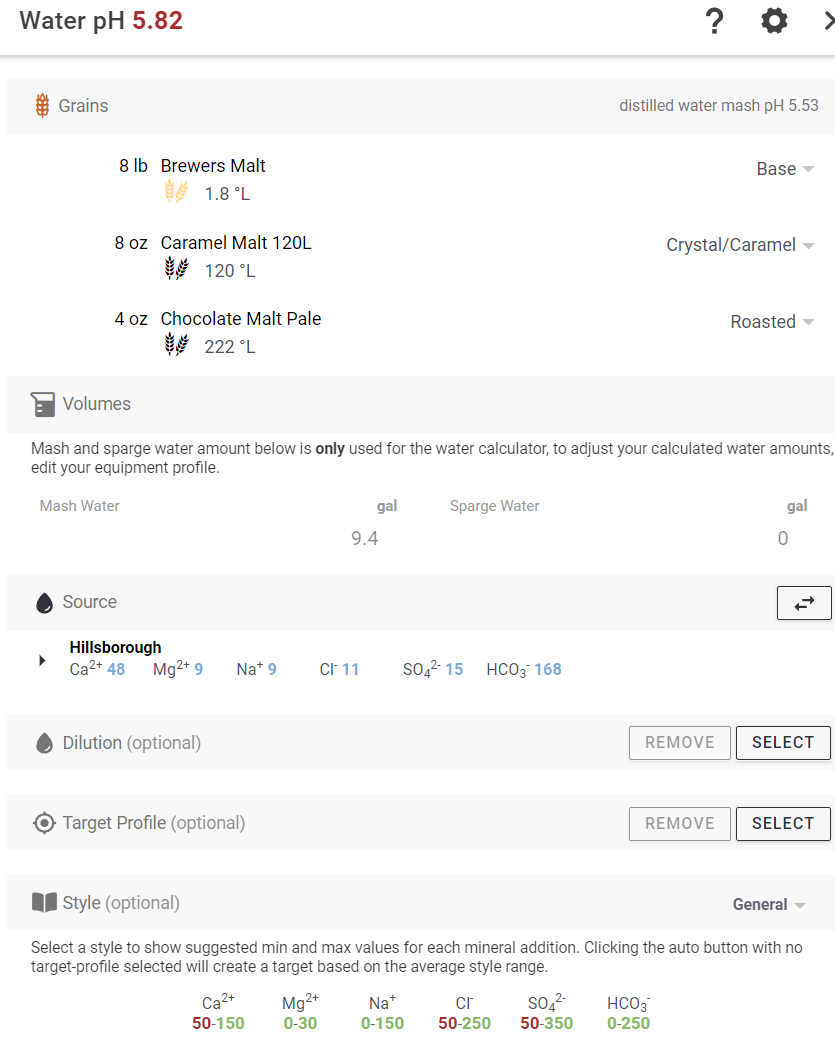
PH = 8.0
TDS = 245 ppm
Electrical conductivity 0.41
Cations / Anions, me/L = 3.5/3.7
Na = 9
K = <1
Ca = 48.4
Mg = 9
CaCO3 = 158
NO3-N = 4.4
SO4-S = 5
Cl = 11
CO3 = <1.0
HCO3 = 168
CaCO3 = 139
P = 0.10
Fe = <1.0
If anybody has any feedback as to if this is a good starting point or not and what direction I should take based on what I have to work with, I'd be very interested in any advice. The beer I've been brewing with it has been good but I am always looking to make it better.
























































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
