Skysharker1
Member
Just tapped my 2 kegs...how long is the waitt time before serving....or is there?? Reg is set at 10psi. Sixtel of Landshark and a sixtel of Shock Top.




Ok.... I'm new to the kegerator world, so pardon any stupid questions I may ask. You say 'already carbonated', so do I need the CO2? I'm trying to understand this whole thing.
Got it...thank you. I did pour a glass from both kegs after I tapped, and they were foamy. That's why I was wondering if there was a wait time perhaps to let the keg settle and equalize with the introduction of the new CO2.Yes, you need the CO2 to push the beer out of the keg, and also to keep the beer fully carbonated, because when you remove beer (thus increasing the headspace), some of the CO2 from the remaining beer would otherwise move into the headspace, reducing the concentration of CO2 in the beer.
Got it...thank you. I did pour a glass from both kegs after I tapped, and they were foamy.
Well, the temp gauge is showing 40° - (I did see the thread about turning the red screw to manipulate the temp settings). The hoses are 3/16" and approx 5' long....and the reg is now showing a bit over 10psi.Your system may not be balanced. (Nothing to do with needing anything to settle.)
You mentioned 10 PSI. What's the temperature of the beer? What's the length and the inside diameter of your beer lines?


Might be that the kegs were recently transported, so they are just big, shaken bottles, that need an hour or so of cold storage to "calm down." Might also be that the beer is warmer than optimal serving temp (35°F - 45°F, depending on personal preference.) Beer that is warm tends to foam more than cold beer when poured.Your system may not be balanced. (Nothing to do with needing anything to settle.)
You mentioned 10 PSI. What's the temperature of the beer? What's the length and the inside diameter of your beer lines?

Thanks!!!!Might be that the kegs were recently transported, so they are just big, shaken bottles, that need an hour or so of cold storage to "calm down." Might also be that the beer is warmer than optimal serving temp (35°F - 45°F, depending on personal preference.) Beer that is warm tends to foam more than cold beer when poured.
Also, as noted many commercially sold kegerators are shipped with lines that are either too short, or too large inside diameter, to provide enough flow resistance to control excess foam during the pour. A basic rule of thumb is that you need about 1foot of 3/16" ID tubing for each psi of serving pressure, so 10 ft for 10 psi. You can get more precise length requirements from here.
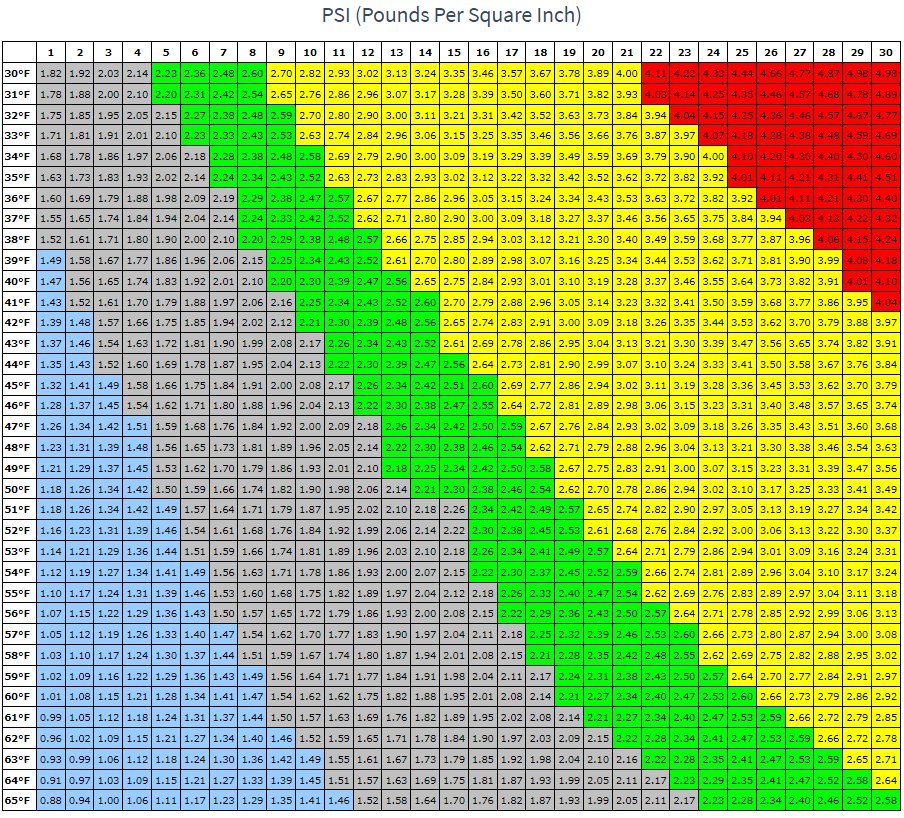
Finally, equilibrium carbonation level is a function of CO2 pressure and temperature. The higher the temperature, the higher the CO2 pressure needs to be to maintain your desired carbonation level. If your pressure isn't right, you will either gain or lose carbonation over time (days and weeks.) You can find a chart for carbonation level as a function of temp and pressure, as well as brief info about carb levels for different styles here, and you can find a calculator here, along with more detailed style vs. carb level info.
Since typical lager and wheat beer carb levels are different, you will have to choose a compromise level that works for you for both of them (unless you get more regulators to provide more different pressures.)
Brew on
Does the hose length apply only to the hose from keg to tap, or the CO2 hose as well??Might be that the kegs were recently transported, so they are just big, shaken bottles, that need an hour or so of cold storage to "calm down." Might also be that the beer is warmer than optimal serving temp (35°F - 45°F, depending on personal preference.) Beer that is warm tends to foam more than cold beer when poured.
Also, as noted many commercially sold kegerators are shipped with lines that are either too short, or too large inside diameter, to provide enough flow resistance to control excess foam during the pour. A basic rule of thumb is that you need about 1foot of 3/16" ID tubing for each psi of serving pressure, so 10 ft for 10 psi. You can get more precise length requirements from here.
Finally, equilibrium carbonation level is a function of CO2 pressure and temperature. The higher the temperature, the higher the CO2 pressure needs to be to maintain your desired carbonation level. If your pressure isn't right, you will either gain or lose carbonation over time (days and weeks.) You can find a chart for carbonation level as a function of temp and pressure, as well as brief info about carb levels for different styles here, and you can find a calculator here, along with more detailed style vs. carb level info.
Since typical lager and wheat beer carb levels are different, you will have to choose a compromise level that works for you for both of them (unless you get more regulators to provide more different pressures.)
Brew on
Just from keg to tap. Length and ID of CO2 lines doesn't make any difference.Does the hose length apply only to the hose from keg to tap, or the CO2 hose as well??

Thank you!! Just redid the hoses. I'll let it sit until tomorrow and give it a whirl.Just from keg to tap. Length and ID of CO2 lines doesn't make any difference.
Brew on
In easy English terms, that means you need to keep the keg at the same pressure to keep it carbonated.fwiw, one thing that consistently escapes folks that are new to kegerators dispensing commercially kegged beers is one needs to provide enough CO2 pressure to maintain the existing carbonation given the keg's new environment (meaning temperature), because if one doesn't put enough pressure on the keg, the beer therein will "out gas" dissolved CO2 until it achieves an equilibrium consistent with said new environment.
And as "bubbles beget bubbles", that outgassing can result in varying degrees of "cascade effect" where a pour results in commensurately foamy content, with the extreme showing a finger or two of liquid at the bottom of a glass of foam. No bueno.
There is science in dispensing beer...
Cheers!
Both those beers are AB products and supposedly all AB products contain 2.7 volumes of CO2. If your kegerator is at 40°, it would require 14-15 psi to maintain 2.7 volumes and at least 15’ (longer wouldn’t hurt a thing) lines to balance the system. You’ll either have to vent the kegs to lower the pressure and let some of the carbonation escape (ie. lowering the volumes) or increase the regulator pressure and beer line length if you want to maintain the same carbonation level they left the brewery at.Well, the temp gauge is showing 40° - (I did see the thread about turning the red screw to manipulate the temp settings). The hoses are 3/16" and approx 5' long....and the reg is now showing a bit over 10psi.
View attachment 816524 View attachment 816525
In easy English terms, that means you need to keep the keg at the same pressure to keep it carbonated.
Enter your email address to join: