Here's a link to an online steam table calculator:
eFunda: Saturated Steam Tables
This assumes saturated steam (ie no superheat), and appears to be working from absolute pressure (psia) as opposed to gauge pressure (psig). 15 psig is about 30 psia (at sea level, anyhow).
If I remember my elementary thermodynamics, you'll be releasing about 945 btu per pound of steam you release into the mash. Thermo geeks: Do I have this right?
[edit]^^^^^
ignore this last bit, you're not releasing steam into the mash directly. You should still be releasing 945 BTU/lb of condensate, but you'd need to know the condensation rate to know how much heat you'd need to supply to the boiler to keep this system working.
Also, please, for your own sake: If you don't have a safety valve in this system, get one. Steam carries a MASSIVE amount of energy, and steam explosions are seriously bad news. See, it's not so much the actual temperature of the steam, but the enthalpy. It takes a lot of energy to make the (l) -> (g) phase change. You get that energy back when you condense the steam, and that's what gives steam heating its power.
Example: Suppose you have steam at 212 F and condense it to water at 212 F. You're not getting a temperature change on condensation, but you'll still release about 897 BTU/lb.
In other words, you don't need to get the system temperature up much above boiling to still be able to transfer a lot of energy to the condenser.
I have personally seen the results of leaks of large quantities of steam superheated to 450F. It ain't pretty. This stuff can be dangerous, so proceed with caution.



![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)






















































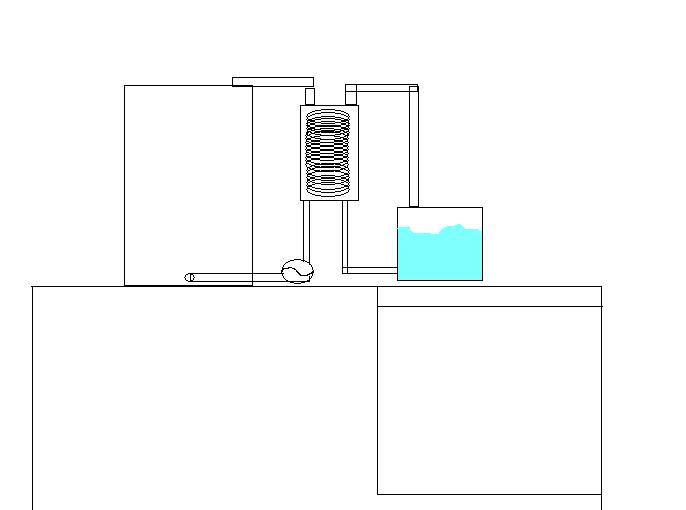
 Please correct me if my assumptions are wrong here.
Please correct me if my assumptions are wrong here.


