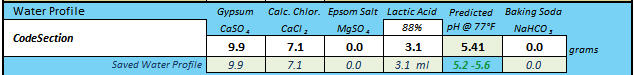
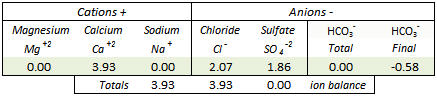
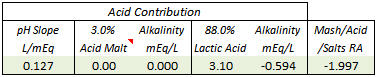
CodeSection
Well-Known Member
- Joined
- Feb 4, 2018
- Messages
- 1,655
- Reaction score
- 819
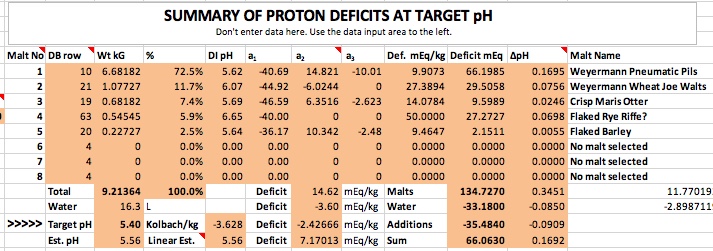
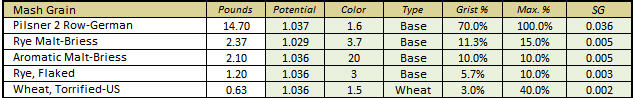
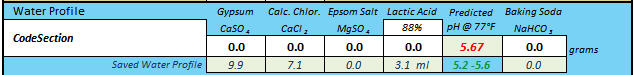
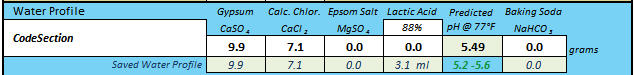
Thank you! Are you interested in taking a DI_pH for your Briess Rye Malt? Mash 50 grams of the malt in 100 mL of distilled (or good quality RO) water at about 146-158 degrees F. for 60 minutes, and take the DI pH reading at room temperature.
Sure, I can do that. Long weekend, wife out of town = extra time.










































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)