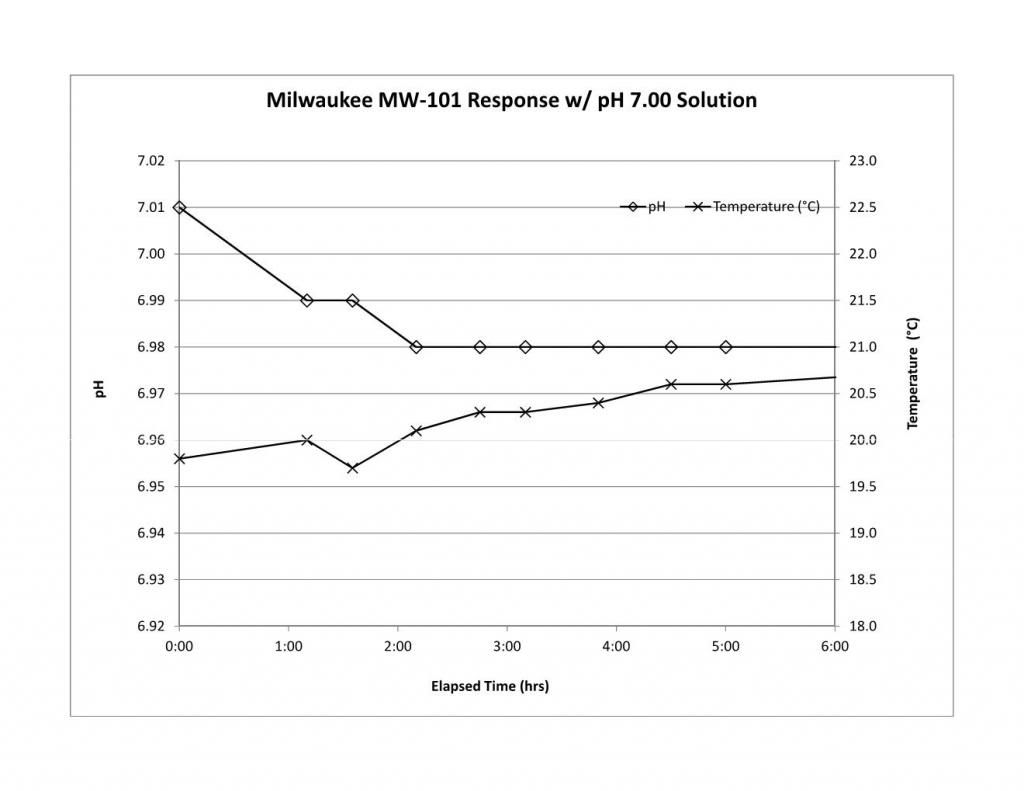
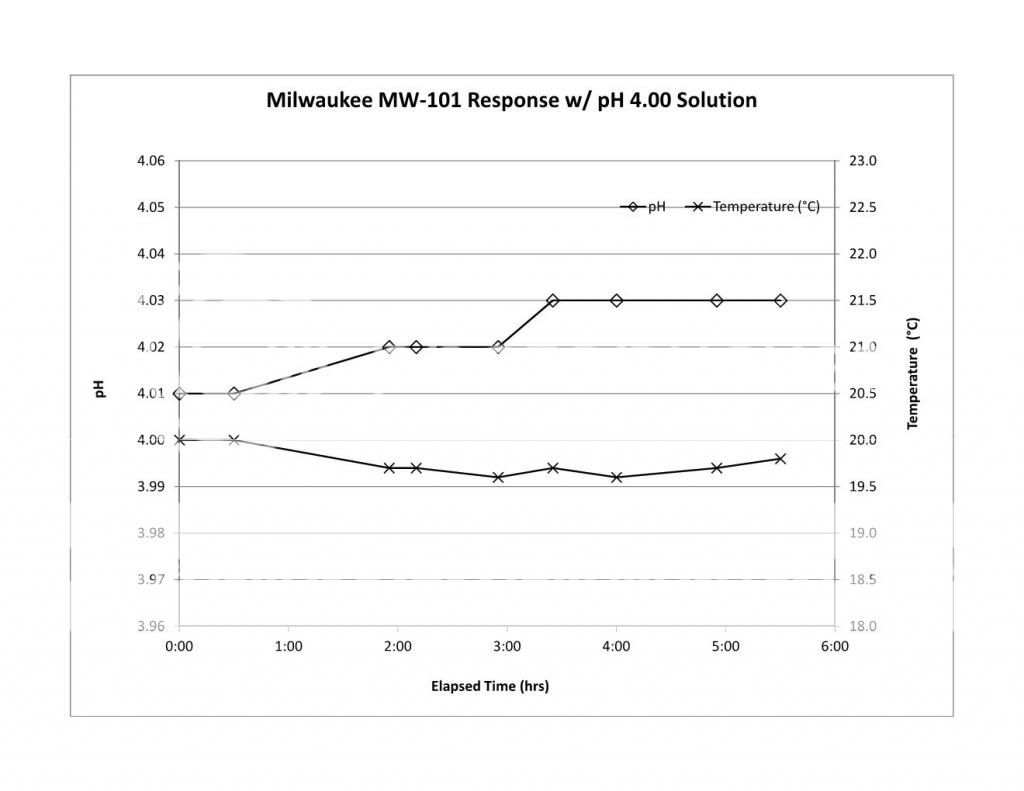
First thing I hope you will do is run the stability check on your new meter. The MW101 has gotten a bad rep mostly for reliability and we hope that the newer design solves the problems.
So. Based on my understanding from this thread, I should heat cal solutions up to a representative mash temp and calibrate at that temp.
While doing the stability check you could heat the buffer and record pH error (the difference between a reported reading and the pH of the buffer - don't forget that buffer pH changes with temperature and you need the actual buffer pH to get the actual error). Now plot the error vs. temperature difference re the calibration temperature i.e. the measurement temperature minus the calibration temperature. The slope of a linear fit to the error vs temperature difference is (pHi - 7)/Tc. Multiplying the slope by Tc, the calibration temperature (in Kelvins) gives the difference between pHi and 7 and so you can estimate pHi for your meter. If it is close to 7 then you may be able to get away without calibrating at near mash temperature. If the line fitting etc. looks overwhelming just looking at the error data will give you an idea. If heating the buffer 20°C results in a pH error of, for example, 0.2 then pHi is too far from 7 and you should cal. at mash temp.
Assuming I do this, I don't have to offset .35, like a room temp reading. Correct?
No, you wouldn't unless you want to compare your pH values to what everyone else is doing. To refer your measured pH to room temperature you would multiply the difference between room temperature and the temp. at which the reading was made (°C) by 0.0055. It would actually be better if you made a series of measurements on a cooling sample in order to determine what the slope for your mashes actually is.
Or would it make sense to just calibrate at room temp, and observe whatever offset I get at mash temp (which would be the .35 plus whatever error is being introduced by the meter so far from it's cal point(s) temps), and just math that in every time?
If you cal at room temp and pHi is close to 7 then you can just take the reading as it is. If pHi not close to 7 then you can figure out what the correction is from (pHi - 7)*delta_T/T_cal (all T's in Kelvins). In other words you can correct the corrections.
At this point, I have ask the deadly question: how much is the probe life shortened. These devices have come a long way since I started using them in the early 90's. Perhaps the probes have as well? After all, it's spec'd to 70 deg C. Will I be the only guinea pig here?
Can't help you there. Is the spec for the ATC or for the electrode? If it is for the ATC I don't understand as the ATC algorithm just pushes numbers and I can't understand why 70 would be a limit. I can understand how the electrode might have a temperature limit, however.









































![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)