troglodytes
Well-Known Member
I've been doing a ton of reading on water over the past year, and now with a lot of help from these forums my beer is definitely better than it previously was. But now as I try to hone in acid additions in different garin bills and styles I am coming across some huge calculated pH discrepancies across different calculators.
I know the best thing I can do is get a pH meter, but it just isn't in the budget right now so sadly I'm just attempting to get the best estimate. I also know that different maltsters will have different DI water pH even when comparing 2-rows or ryes; however the discrepancies I'm seeing would, in my opinion, be the difference between an ok beer and a ruined one, so I just wanted to get an opinion.
Below is my grain bill and water adjustments from Bru'n water (what I always use to determine my additions). This is for a Rye IPA ( a fairly big one).

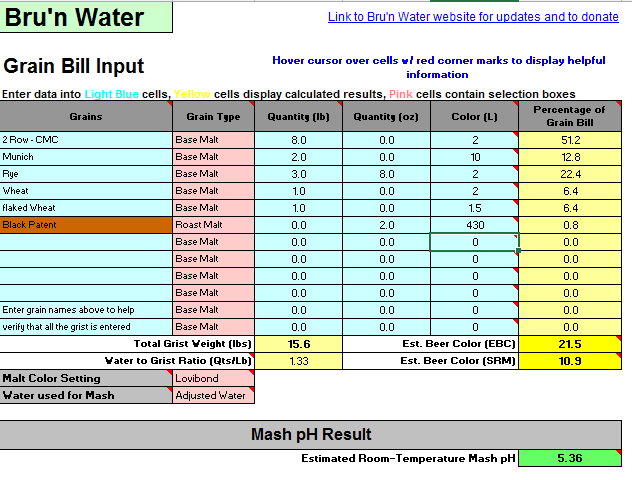
Now if I enter this exact data in Brewer's Friend and EZ Water I get the same results between those 2; however; they are very far off on pH from Bru'n water. Both BF and EZ say I need at a minimum 6 mL of lactic acid to get to 5.3 mash pH. This seems very high to me so I entered it into Bru'n water to see what I would get. According to Bru'n if I added 6 mL of lactic to my mash I would be at a mash pH of 4.8 which I think is pretty much unacceptable.
What would you do in this situation?
I know the best thing I can do is get a pH meter, but it just isn't in the budget right now so sadly I'm just attempting to get the best estimate. I also know that different maltsters will have different DI water pH even when comparing 2-rows or ryes; however the discrepancies I'm seeing would, in my opinion, be the difference between an ok beer and a ruined one, so I just wanted to get an opinion.
Below is my grain bill and water adjustments from Bru'n water (what I always use to determine my additions). This is for a Rye IPA ( a fairly big one).


Now if I enter this exact data in Brewer's Friend and EZ Water I get the same results between those 2; however; they are very far off on pH from Bru'n water. Both BF and EZ say I need at a minimum 6 mL of lactic acid to get to 5.3 mash pH. This seems very high to me so I entered it into Bru'n water to see what I would get. According to Bru'n if I added 6 mL of lactic to my mash I would be at a mash pH of 4.8 which I think is pretty much unacceptable.
What would you do in this situation?











































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)



