ocwo92
Well-Known Member
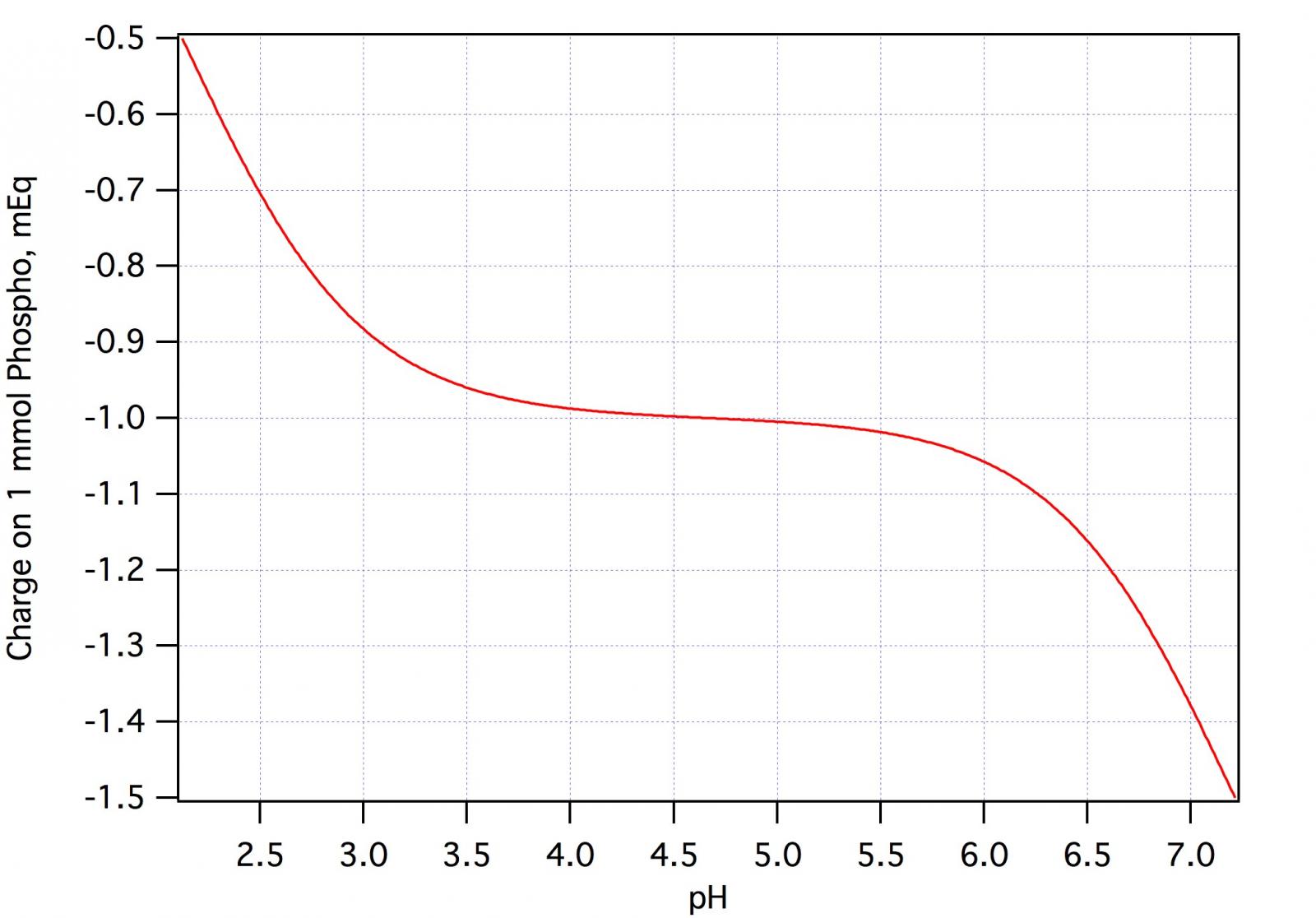
I've seen a few threads (notably, the sticky one on all grain) mentioning that the pH 5.2 stabilizer doesn't work. Several people seem to agree.
How did you/they reach that conclusion?
(The easy answer might be that they added the proper amount of stabilizer to the mash water and then measured the pH value. However, the stabilizer is supposed to react with the malt, so measuring the pH value of water with the stabilizer wouldn't work.)
How did you/they reach that conclusion?
(The easy answer might be that they added the proper amount of stabilizer to the mash water and then measured the pH value. However, the stabilizer is supposed to react with the malt, so measuring the pH value of water with the stabilizer wouldn't work.)