boarderx3120
Active Member
- Joined
- May 27, 2016
- Messages
- 34
- Reaction score
- 15
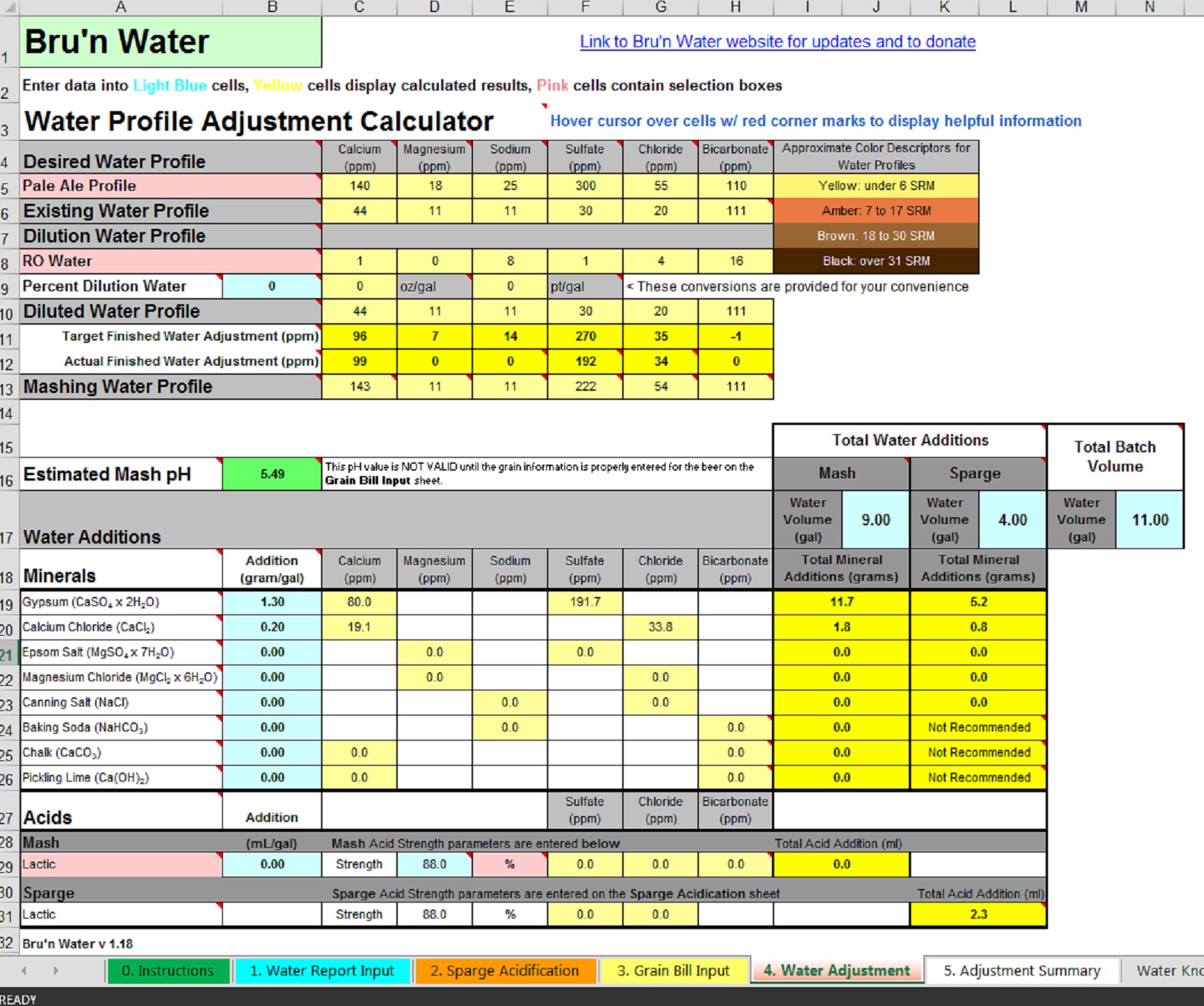
I've been slowing dipping my toes in the idea of water chemistry lately so I got a report from my municipal water source and put in all the relative information into the Bru'n Water spreadsheet, however I am confused on the additions. In an attempt to keep it simple, I typed in 1.3g of gypsum per gallon of mash and sparge water. Now I am at the higher end of the calcium range (as I understand it) but still quite low in the sulfate range. How do I get that sulfate value up? It appears Epsom salt is an option (?) but that isn't something I typically hear brewers are adding to their water. Also, what is up with that bicarbonate level. Should I be concerned with that? Here is a screen shot of what I am seeing. Your thoughts are appreciated.








![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)

















































