You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
My CO2 tank reads low gas when cold
- Thread starter meylo
- Start date

Help Support Homebrew Talk:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
user 125772
Well-Known Member
- Joined
- Jul 18, 2012
- Messages
- 941
- Reaction score
- 272
expected behavior
Yes, this is normal behavior. You need to touch up the regulator setting as well.
Cheers!
GoeHaarden
The best advice is unsolicited
- Joined
- Feb 1, 2017
- Messages
- 1,359
- Reaction score
- 768
Gas laws. Pressure is directly related to temperature...
This makes sense. So please correct me here. The reading shows lower because of the temp change right? So do I need to make any adjustments to the PSI meter to compensate in any way or should I trust the PSI reading level (20 psi)? FYI my regulators max setting is 30 PSI.

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
Guangshui Weilu You Trading Co., Ltd

$33.99 ($17.00 / Count)
$41.99 ($21.00 / Count)
2 Pack 1 Gallon Large Fermentation Jars with 3 Airlocks and 2 SCREW Lids(100% Airtight Heavy Duty Lid w Silicone) - Wide Mouth Glass Jars w Scale Mark - Pickle Jars for Sauerkraut, Sourdough Starter
Qianfenie Direct

$22.00 ($623.23 / Ounce)
AMZLMPKNTW Ball Lock Sample Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging joyful
无为中南商贸有限公司

$172.35
2 Inch Tri Clamp Keg Manifold With Ball Lock Posts, Pressure Gauge, PRV (0-30 PSI) – Homebrew, Fermentation, Kegging System
wuhanshijiayangzhiyimaoyiyouxiangongsi

$20.94
$29.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.com
![Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]](https://m.media-amazon.com/images/I/41fVGNh6JfL._SL500_.jpg)
$6.95 ($17.38 / Ounce)
$7.47 ($18.68 / Ounce)
Craft A Brew - Safale S-04 Dry Yeast - Fermentis - English Ale Dry Yeast - For English and American Ales and Hard Apple Ciders - Ingredients for Home Brewing - Beer Making Supplies - [1 Pack]
Hobby Homebrew

$53.24
1pc Hose Barb/MFL 1.5" Tri Clamp to Ball Lock Post Liquid Gas Homebrew Kegging Fermentation Parts Brewer Hardware SUS304(Gas MFL)
yunchengshiyanhuqucuichendianzishangwuyouxiangongsi

$58.16
HUIZHUGS Brewing Equipment Keg Ball Lock Faucet 30cm Reinforced Silicone Hose Secondary Fermentation Homebrew Kegging Brewing Equipment
xiangshuizhenzhanglingfengshop

$10.99 ($31.16 / Ounce)
Hornindal Kveik Yeast for Homebrewing - Mead, Cider, Wine, Beer - 10g Packet - Saccharomyces Cerevisiae - Sold by Shadowhive.com
Shadowhive

$39.22 ($39.22 / Count)
Brewer's Best Home Brew Beer Ingredient Kit - 5 Gallon (Mexican Cerveza)
Amazon.com

$7.79 ($7.79 / Count)
Craft A Brew - LalBrew Voss™ - Kveik Ale Yeast - For Craft Lagers - Ingredients for Home Brewing - Beer Making Supplies - (1 Pack)
Craft a Brew

$176.97
1pc Commercial Keg Manifold 2" Tri Clamp,Ball Lock Tapping Head,Pressure Gauge/Adjustable PRV for Kegging,Fermentation Control
hanhanbaihuoxiaoshoudian

$719.00
$799.00
EdgeStar KC2000TWIN Full Size Dual Tap Kegerator & Draft Beer Dispenser - Black
Amazon.com
You may experience some drift, typically downwards, if you stick a regulator in a fridge/keezer and it chills down.
Otherwise, note even at 32°F temperature and a nearly empty cylinder, the vapor pressure is at least a few hundred PSI above your regulator setting...
Cheers!
Otherwise, note even at 32°F temperature and a nearly empty cylinder, the vapor pressure is at least a few hundred PSI above your regulator setting...
Cheers!
kevinthestout
Member
- Joined
- Oct 14, 2017
- Messages
- 21
- Reaction score
- 8
That would be true if you were dealing with a fixed volume of gas, but under normal storage conditions CO2 liquefies. For a given temperature the gas pressure is limited by the pressure at which the gas liquefies. At 0°C the liquefaction pressure is ~12.5bar (181psi) . At 20°C the liquefaction is pressure is 60bar (870psi). Once the tank is sufficiently empty that it can't reach liquefaction pressure then the pressure will be approximately proportional to temperature.Gas laws. Pressure is directly related to temperature...
user 125772
Well-Known Member
- Joined
- Jul 18, 2012
- Messages
- 941
- Reaction score
- 272
So do I need to make any adjustments to the PSI meter to compensate in any way or should I trust the PSI reading level (20 psi)?
No, you don’t adjust the regulator, but Are you telling us you serve beer at 20 psi ?
FloppyKnockers
Well-Known Member
No, you don’t adjust the regulator, but Are you telling us you serve beer at 20 psi ?
No. I am carbonating over time.
GoeHaarden
The best advice is unsolicited
- Joined
- Feb 1, 2017
- Messages
- 1,359
- Reaction score
- 768
At 0°C the liquefaction pressure is ~12.5bar (181psi) . At 20°C the liquefaction is pressure is 60bar (870psi).
Soooo....Like I said, and like you reiterated here^^. Pressure is directly related to temperature. Considering our CO2 is evaporating from liquid to gas we aren't concerned with liquification unless we are filling our tanks from a gaseous CO2 into liquid CO2. Even so, the relationship still exists.
kevinthestout
Member
- Joined
- Oct 14, 2017
- Messages
- 21
- Reaction score
- 8
Soooo....Like I said, and like you reiterated here^^. Pressure is directly related to temperature.
Yes, pressure is related to temperature, but in this case not by any of the gas laws. Which gas law were you thinking of?
As a senior member of the Dead Horse Committee I will keep this going and likely demonstrate my ignorance on the subject at the same time.
Regarding the original question; the pressure in the CO2 bottle is controlled by the vapor pressure unless there is no liquid CO2 left.
What I experience as well is that the line pressure after the regulator drops as well (when the entire bottle and regulator is the cold box along with the beer keg). I suspect this is due to the regulator design and its temperature coefficient rather than the temperature of the gas. Also, the pressure gauges likely have their own temperature coefficients as well further complicating the issue.
Regarding the original question; the pressure in the CO2 bottle is controlled by the vapor pressure unless there is no liquid CO2 left.
What I experience as well is that the line pressure after the regulator drops as well (when the entire bottle and regulator is the cold box along with the beer keg). I suspect this is due to the regulator design and its temperature coefficient rather than the temperature of the gas. Also, the pressure gauges likely have their own temperature coefficients as well further complicating the issue.
GoeHaarden
The best advice is unsolicited
- Joined
- Feb 1, 2017
- Messages
- 1,359
- Reaction score
- 768
Yes, pressure is related to temperature, but in this case not by any of the gas laws. Which gas law were you thinking of?
Well, Guy-Lussac's law explains this direct relationship between temperature and pressure of a gas at a constant volume. Go stick your CO2 tank in the fridge and compare the pressure readings to when it is at room temp. A cold CO2 tank will show lower pressures than that of one at room temp. I think you're getting tripped up by the fact that CO2 exists as a liquid and gas in our tanks. Even so, liquids will also expand as temperature increases and thus pressure will increase at constant volume. I will say it again, a direct relationship exist between temperature and pressure.
What point were you trying to make with your initial response?
kevinthestout
Member
- Joined
- Oct 14, 2017
- Messages
- 21
- Reaction score
- 8
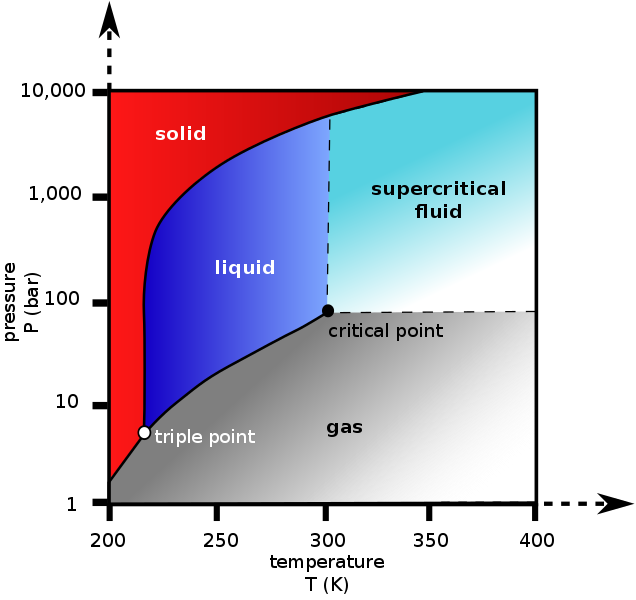
Guy Lussac's Law states that pressure is proportional to absolute temperature. In this case it is not. The pressure is due to the phase change. You can see how the pressure is related from the phase diagram. We're in agreement that pressure is related to temperature it's just nothing to do with gas laws.Well, Guy-Lussac's law explains this direct relationship between temperature and pressure of a gas at a constant volume. Go stick your CO2 tank in the fridge and compare the pressure readings to when it is at room temp. A cold CO2 tank will show lower pressures than that of one at room temp. I think you're getting tripped up by the fact that CO2 exists as a liquid and gas in our tanks. Even so, liquids will also expand as temperature increases and thus pressure will increase at constant volume. I will say it again, a direct relationship exist between temperature and pressure.
What point were you trying to make with your initial response?
kevinthestout
Member
- Joined
- Oct 14, 2017
- Messages
- 21
- Reaction score
- 8
Regarding the original question; the pressure in the CO2 bottle is controlled by the vapor pressure unless there is no liquid CO2 left.
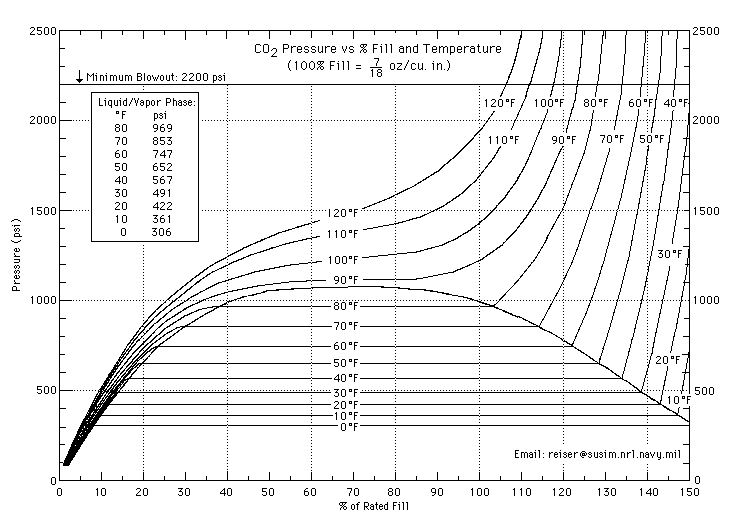
That (and what I said previously) is almost true. The chart that day_trippr posted shows that above the critical temperature (31.1 °C) all the CO2 in the tank is a supercritical fluid and starts to behave like a gas. This means that above 31.1 °C you can tell how full the tank is from the pressure. Also from the gradient of the contours on the far left and far right of that chart you can see that liquid CO2 is almost as compressible as CO2 gas.
https://www.homebrewtalk.com/forum/members/day_trippr.81618/
I've flogged more dead horses than Findus.
I don't really understand the science in kevinthestout's chart, but I do know that now I'm going to call my next brew "supercritical fluid"
Silver79
Well-Known Member
My super basic and not total accurate way of remembering this stuff goes back to my 10th grade chemistry teacher who used to say "pv=nrt it's not just a good idea, it's the law". Since it's the ideal gas law it doesn't really apply but always reminds me to consider pressure and volume in relation to temperature.
- Joined
- May 6, 2015
- Messages
- 61
- Reaction score
- 45
Henry's law can also help in understanding this scenario. It pretty much quantifies what was mentioned above with partial pressures with simple equations. The CO2(gas) inside of your canister will dissolve more readily into the CO2(liquid) inside of the container as temperature drops, leaving a lower pressure in the headspace.
bracconiere
Jolly Alcoholic - In Remembrance 2023
That gauge with the green and red is pretty much worthless.
+1, if it drops, time to swap.....
edit: as long as the temp is relatively stable.....
Last edited:
wsmith1625
Well-Known Member
While we're on the subject, I recall reading threads where people said that storing the co2 tank in the refrigerator will make the tank last longer. I never understood this, and don't know if it's even true. Does anyone care to explain? I have always kept my tank outside the fridge because of space issues.
jseyfert3
Well-Known Member
There's no truth to that. The mass of CO2 used has no relation to the temperature the CO2 is stored at. It will last the same stored in or out of the fridge.While we're on the subject, I recall reading threads where people said that storing the co2 tank in the refrigerator will make the tank last longer. I never understood this, and don't know if it's even true. Does anyone care to explain? I have always kept my tank outside the fridge because of space issues.
wsmith1625
Well-Known Member
There's no truth to that. The mass of CO2 used has no relation to the temperature the CO2 is stored at. It will last the same stored in or out of the fridge.
That's what I thought. Thanks!
bracconiere
Jolly Alcoholic - In Remembrance 2023
That's what I thought. Thanks!
i guess if you keep your kegs at room temp, might be able to push a couple extra......co2 does expand when it's warmer.....lol
wsmith1625
Well-Known Member
My wife drinks hey seltzer at room temperature. Every time she opens a new bottle I think to myself ”all your co2 is escaping”. 
bracconiere
Jolly Alcoholic - In Remembrance 2023
but co2 escaping is what gives it bubbles!
wsmith1625
Well-Known Member
I know, but I compare everything to beer. Try pouring a warm beer from the tap. The co2 escapes pretty quickly in your glass of foam
bracconiere
Jolly Alcoholic - In Remembrance 2023
i've tried to deal with that before.....no luck....
Similar threads
- Replies
- 1
- Views
- 2K
- Replies
- 0
- Views
- 1K
Latest posts
-
-
-
Need help diagnosing lower than expected efficiency
- Latest: micraftbeer
-
-
-
Side quest! Hobby talk including beekeeping
- Latest: Sailingeric














































