kingschiff
Well-Known Member
- Joined
- Oct 20, 2018
- Messages
- 99
- Reaction score
- 0
I can only vouch that induction burners throw off electrical measurements rather effectively.
I have gas/flame burners

I can only vouch that induction burners throw off electrical measurements rather effectively.
I agree with Lawrence that there is little chance for that grist to drive pH down as far as reported. There must be something out of sorts with your pH measuring equipment.
Your mineral additions are healthy, but not crazy. So that shouldn't drive pH down that far.
GRAINS: 10 LB pale malt, 1 lb flaked oats, 1 lb flaked red wheat
MASH SALTS (5.8g Cal chl, 2.4g gypsum, .4 table, .4 epsom) into 4.25 gallons distilled water
PH (temp of reading)
MASH WATER+SALTS PH- 5.7 (72.8*)
15 MIN AFTER DOUGH: 5.2 (69.6)
30 min after dough: 4.9 (72.3)
end of mash: 4.9 (71.5)
SPARGE WATER+ SALTS PH: 5.8 (71.1) (3.25 gal, 5g cal chl, 2.1g gypsum, .3 table, .3 epsom)
SPARGE RUNOFF PH: 5 (72)
FULL WORT PH: (4.6)
PH meter was checked last week with distilled/baking soda solution, milk, and PH solution. All worked. Checked prior to starting brewing with PH solutions. Now i did have a 73% efficiency which is better than what I've been getting. Also a IPA i was drinking this weeknd had a PH of 4.2 (as i just happened to check as I was brewing)
But this MASH PH thing is confusing the hell out of me. BruN had 5.32, MME 5.69 and Kaiser's sheet at 5.55 (with an avg of 5.52)
I am 100% sure (almost) I am not committing any errors or wrong doings.
What the heck do you guys think the issue is? Is there even an issue here? Thanks again for any comments
Your pH meter is off by more than I originally presumed. I initially misread the two wheats to be malts, but they are actually flaked. Flaked has a much higher DI_pH than does malted. There has been no level of applied kilning to develop acidity.
These are OK for sanity checks but not for checking the calibration of the meter.PH meter was checked last week with distilled/baking soda solution, milk, and PH solution. All worked.
What is a "pH solution?"Checked prior to starting brewing with PH solutions.
Those numbers should grant you some perspective on the worth of mash prediction calculators.But this MASH PH thing is confusing the hell out of me. BruN had 5.32, MME 5.69 and Kaiser's sheet at 5.55 (with an avg of 5.52)
Your sin is one of omission. But don't spend the afternoon on Hail Ninkasi's. Learning to use a pH meter properly takes some time, practice and understanding. It is an art. You have not checked your pH meter according to the protocol in the Sticky. It appears to be drifting fast. The pH of your mash should be quite high (5.7 - 5.8 depending on the properties of the grains). The salts you added will have small effect. Using 3.5 as the Kolbach factor the CaCl2 would pull the pH down by 0.058, the gypsum by 0.016 and the epsom salts by 0.001. The actual drops will probably be about half that.I am 100% sure (almost) I am not committing any errors or wrong doings.
What the heck do you guys think the issue is? Is there even an issue here? Thanks again for any comments
My worry is shelling out for a new meter and figuring out it's not that.
Although at this point it really seems like the only variable.










There are some who will try to tell you that you don't need ATC but you really do. In fact you can get by without it but having it relieves you of having to do some things and worry about others.
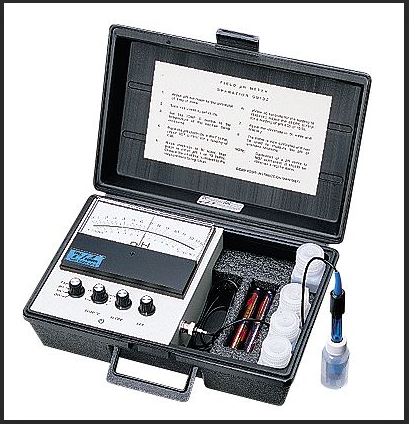
See.
Now Martin has never understood this the myriad times it's been explained before so I doubt he will this time but we are not concerned about what he understands here so much as we .....If you buy a modern meter you are getting ATC whether you appreciate its virtues or not!
The MW102 is a fine meter for brewing. Milwaukee is in the "hobbyist" class of supplier. You can move up a step to a laboratory/industry level supplier by going with the Hach or Omega pocket meters for around $125. But I cannot point to anything that the extra $20 buys you. Also some people just prefer the probe configuration to the pen configuration. In the former any electrode with a BNC connector can be used to replace the original probe when it (as it eventually will) fails. With the pen types you must go with the manufacturers' replacement electrodes. These often cost half or more of the price of a new meter.
On HomeBrewTalk the MW102, Hach pHPro+ and the Omega are considered vetted. This is a little unfair as over time other manufacturers have developed meters that are as good. As no one uses them here they don't get talked about much.
On HomeBrewTalk the MW102, Hach pHPro+ and the Omega are considered vetted. This is a little unfair as over time other manufacturers have developed meters that are as good. As no one uses them here they don't get talked about much.
Which others?, I’m ready to buy a new meter.
If I knew I'd tell you. From time to time someone will post a "How about the pHisting 117B? Has anyone used that?" The answer is always "Don't know anything about it. Specs look OK. Why don't you get one, do the stability check on it and let us know." No one has ever done that to the extent that we could add another meter to the list and until someone does were stuck with the three that people have done that for.Which others?