North_of_60
Well-Known Member

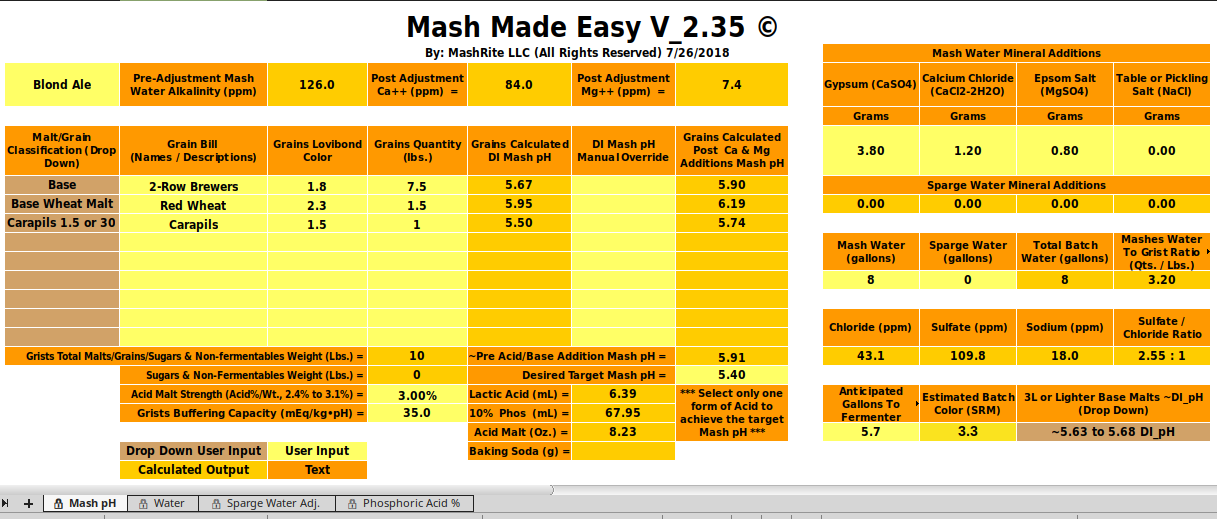
This is my first time adding acid to my mash. It’s a Blonde Ale, the recipe and MME numbers are attached.
Is 7.08 ml a lot of lactic acid for an 8 gallon BIAB full volume mash for a 5 gallon batch? Am I going to get a sour taste?
At what point should I consider using phosphoric acid?
This Blonde is the lightest beer that I make. My Oatmeal Stout only calls for 2.76 ml of lactic acid. My stouts and porters taste pretty good with no additions. Though, I think lowering the pH will help them be even better. My Irish Red was ok but definitely could have used some help. So if I can be ok with 7 ml lactic acid in my Blonde I will only have to keep one kind of acid in the house. I like to keep things simple. Thanks.















































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)