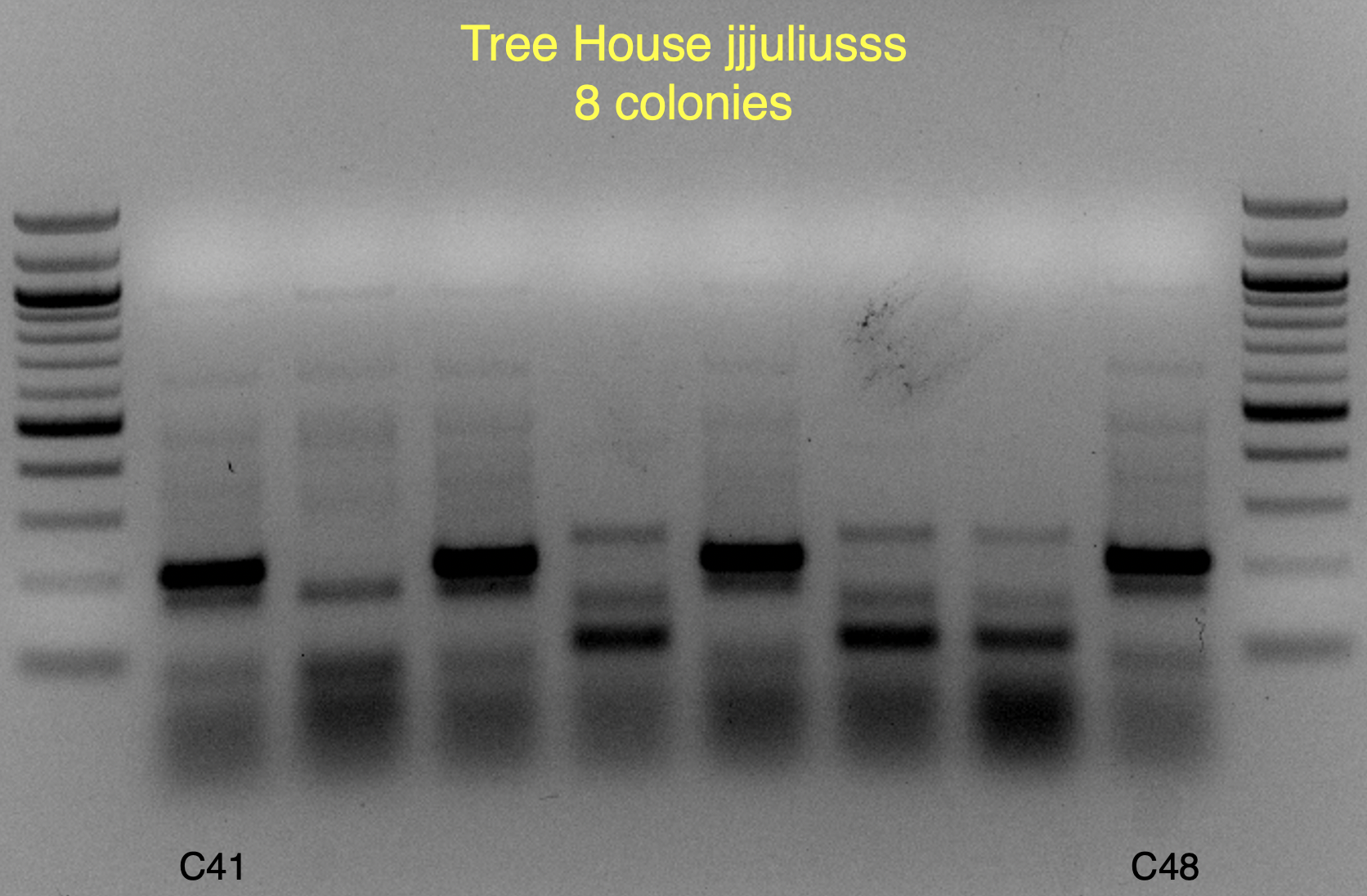
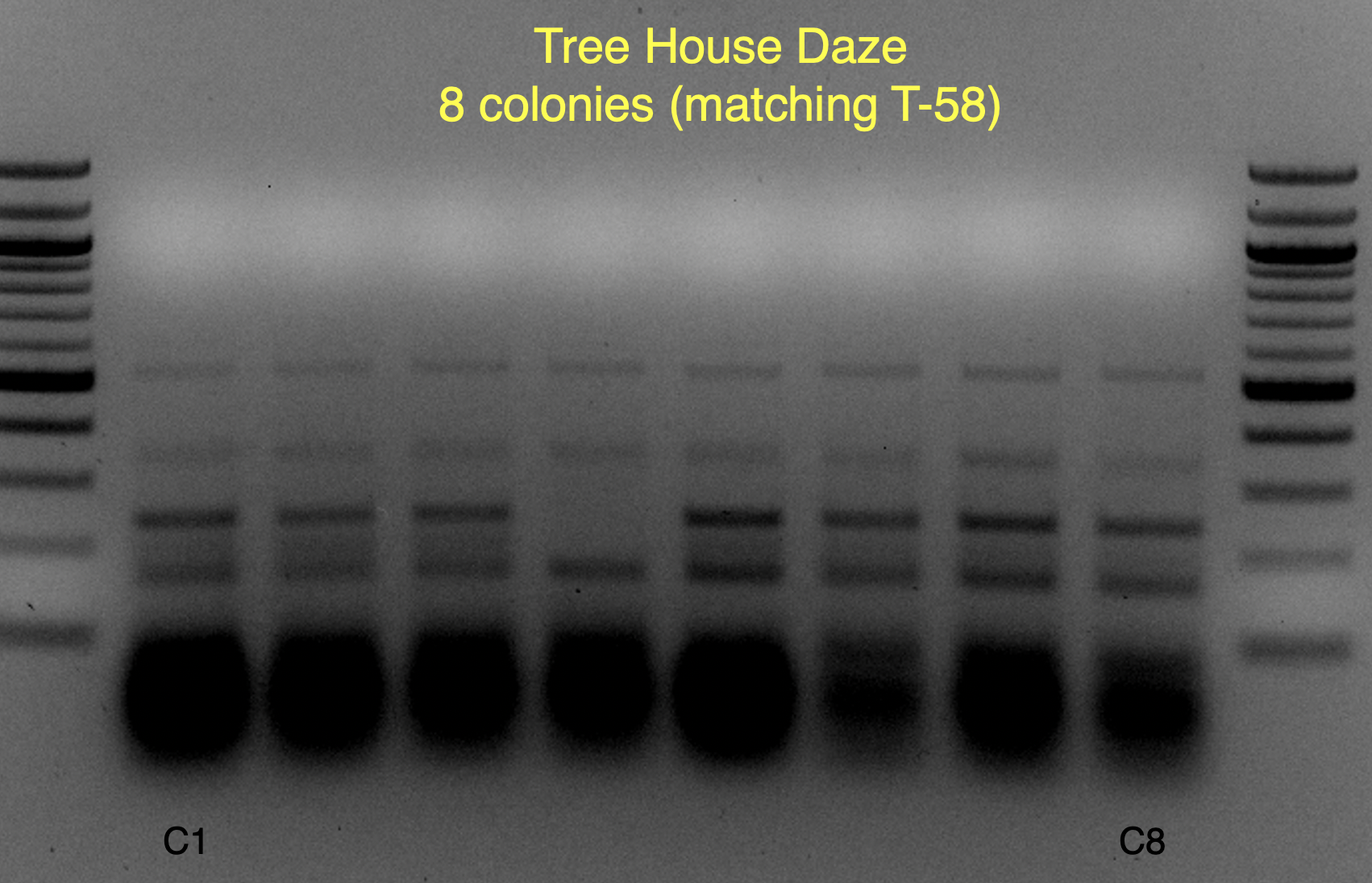
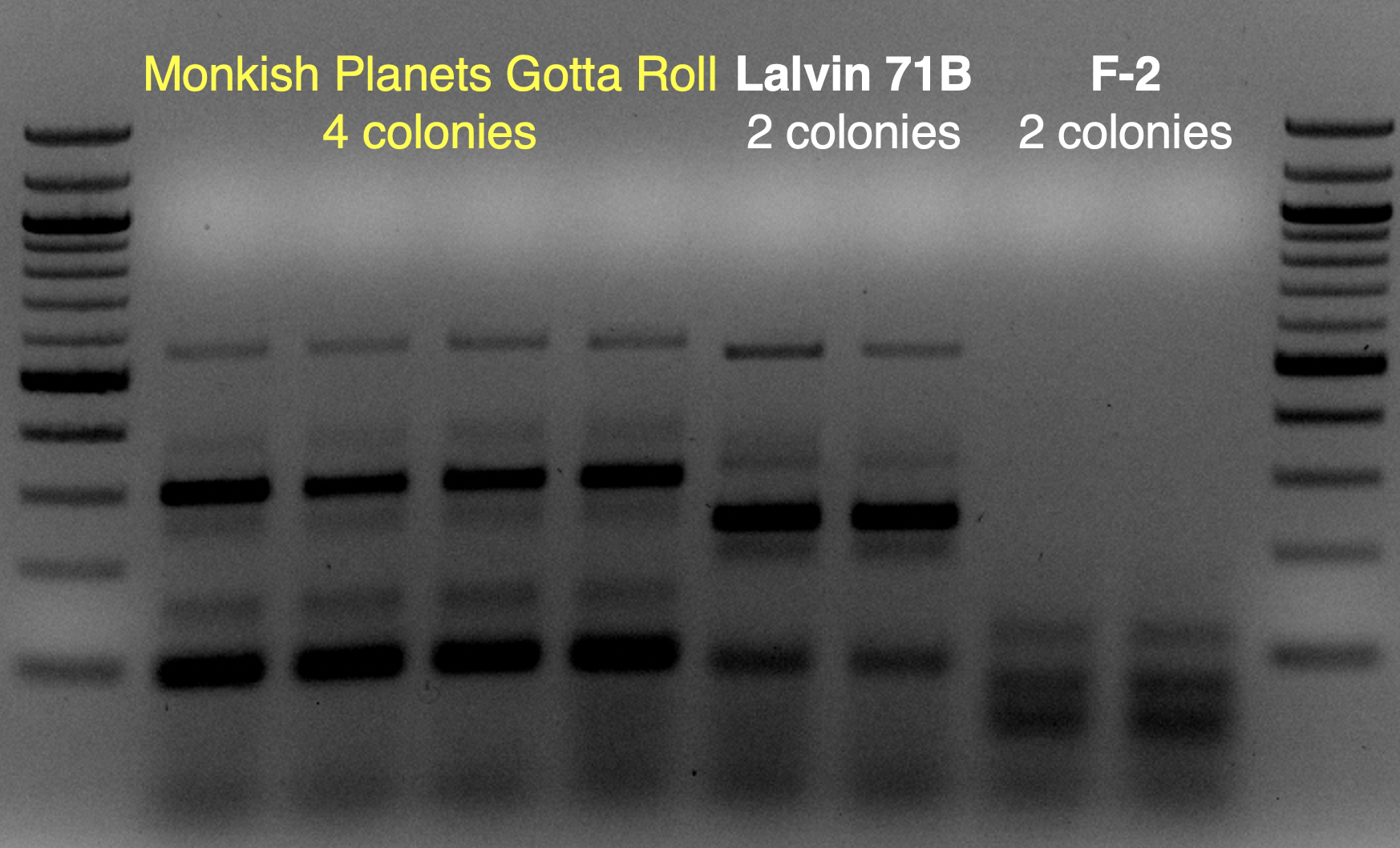
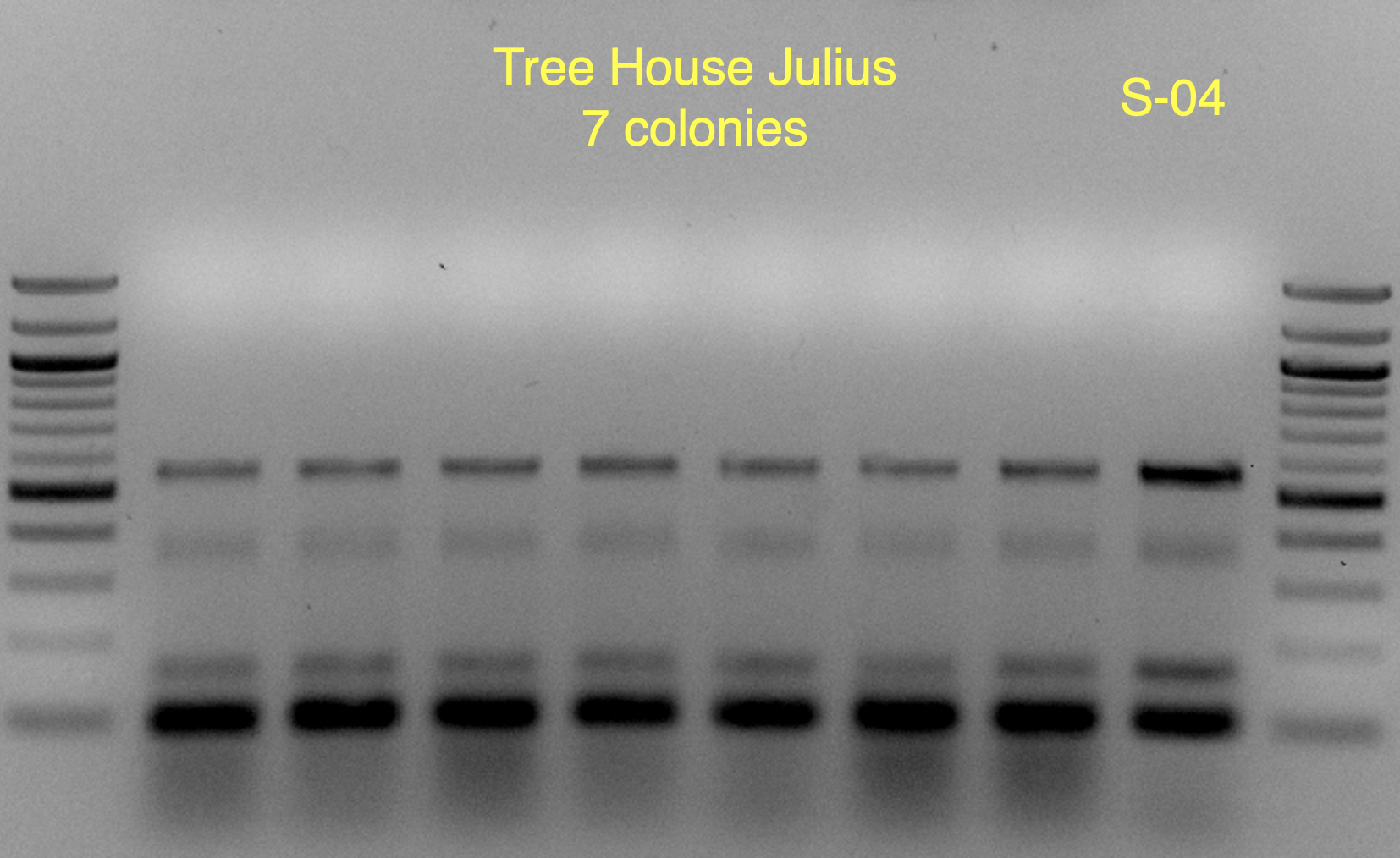
View attachment 699349
View attachment 699350
View attachment 699351
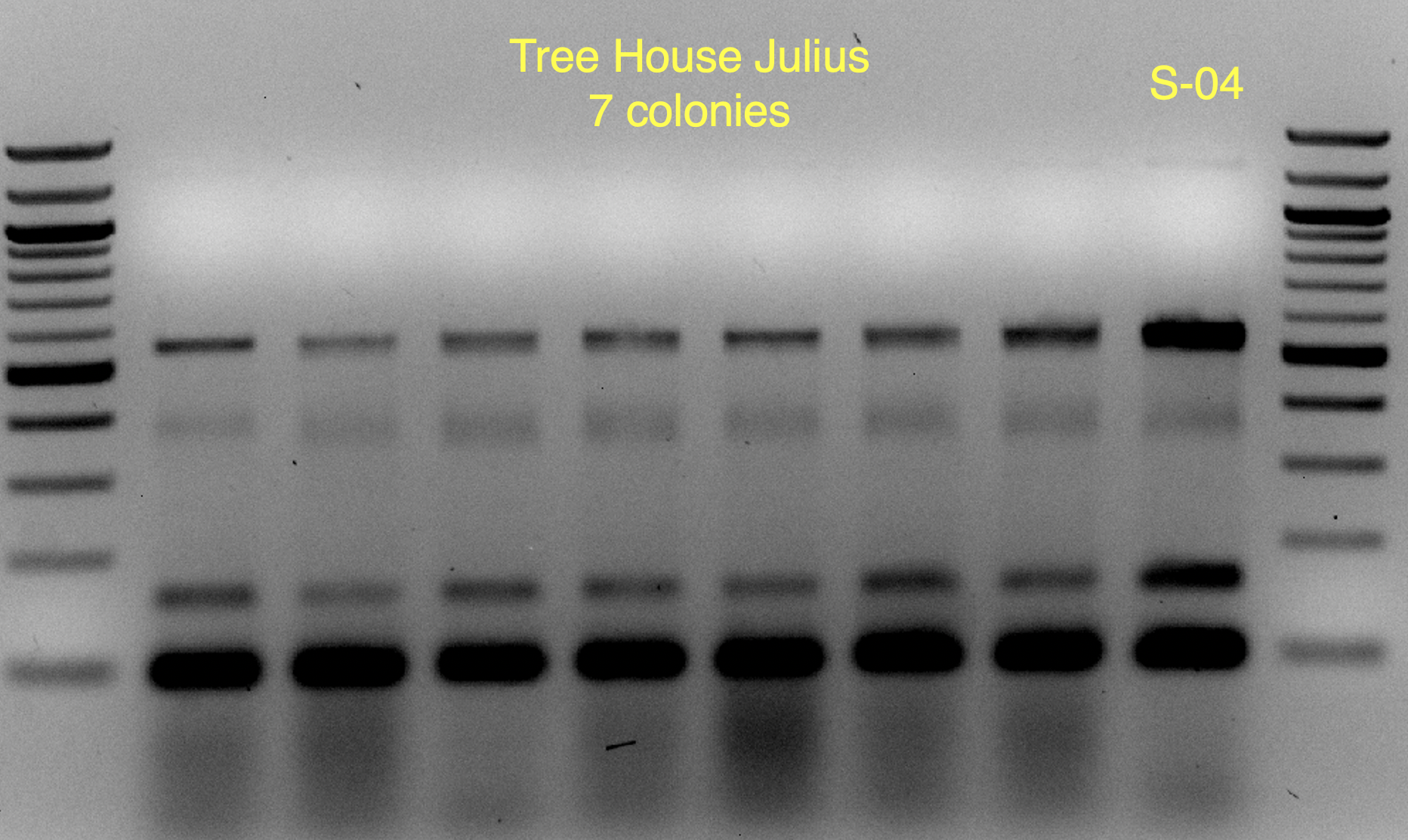
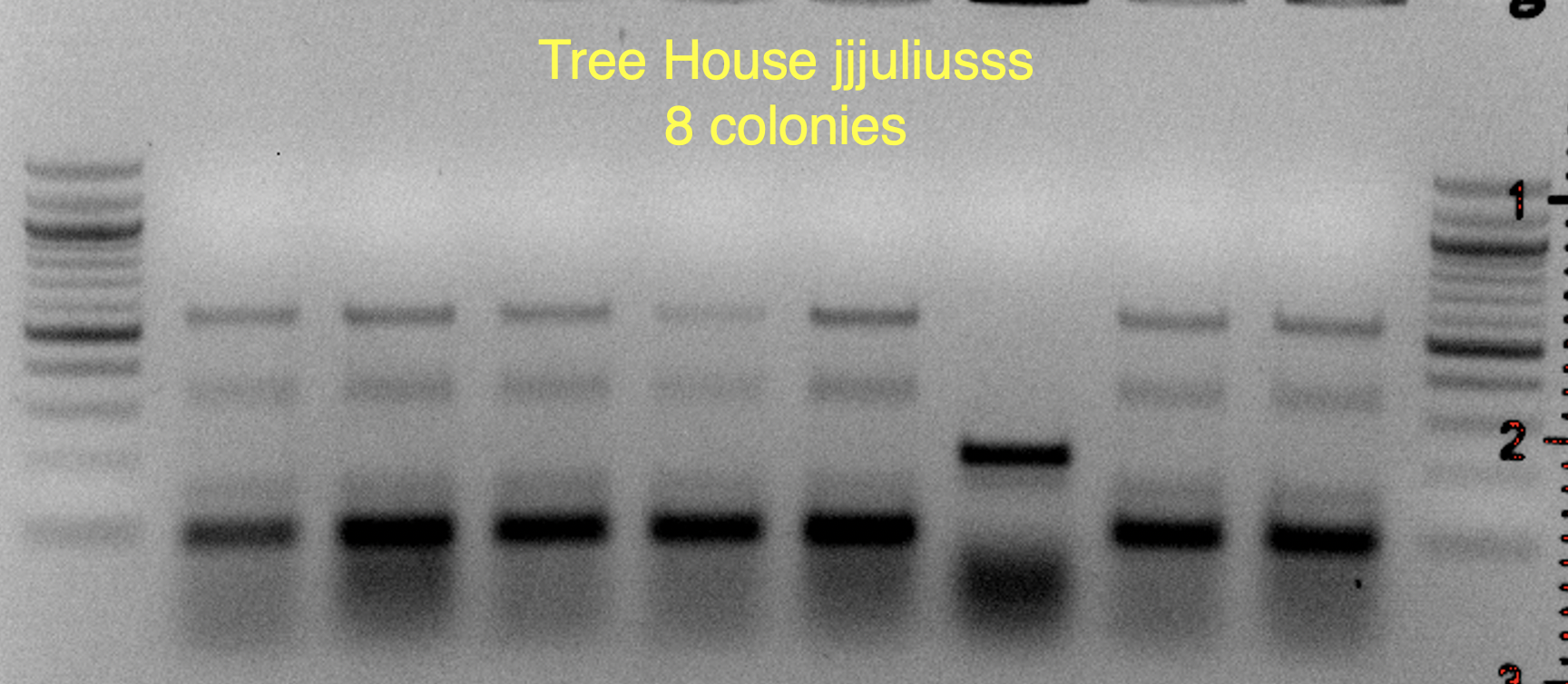
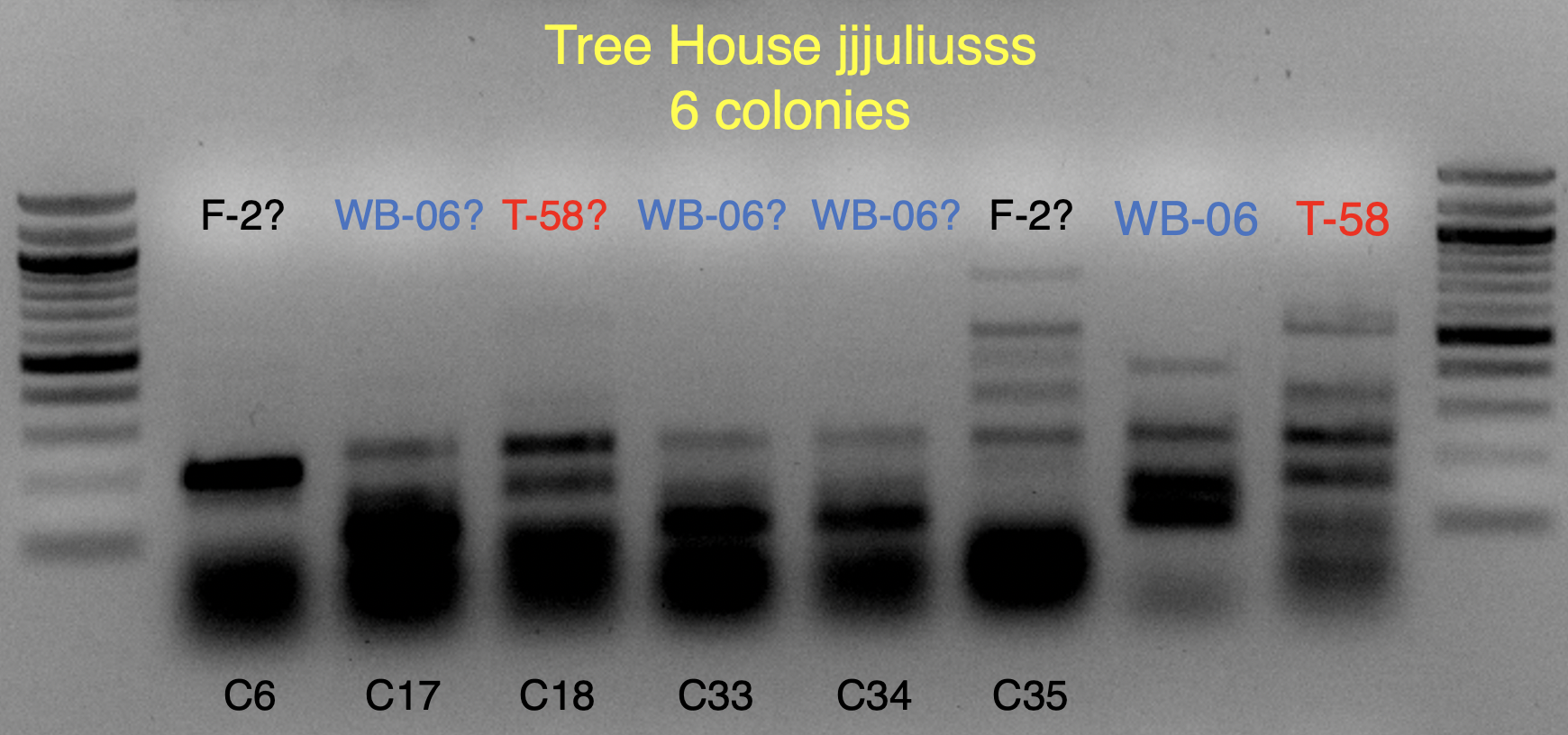
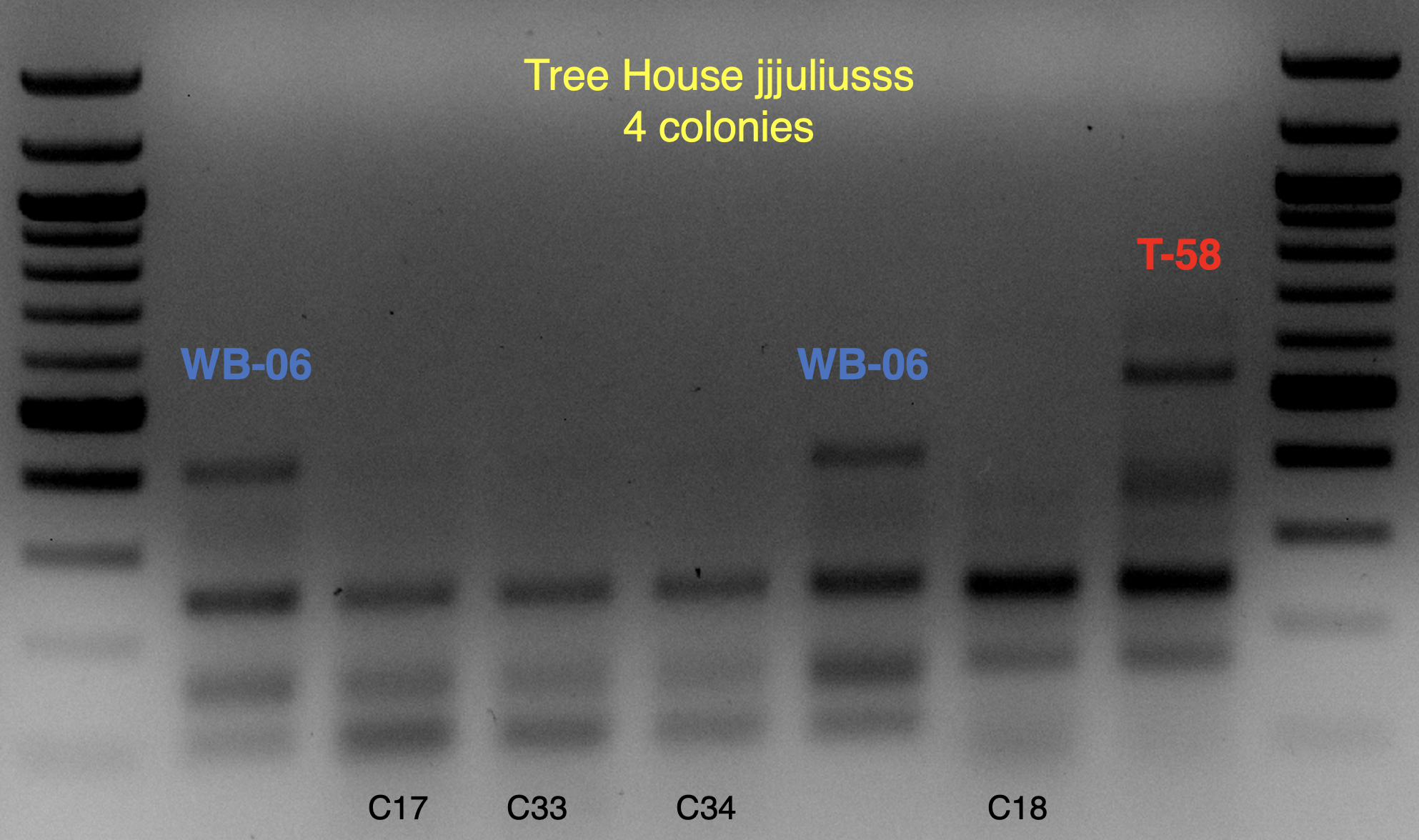
These strains are likely WB-06 and T-58 (as identified by
@isomerization), but I’ll say these are unconfirmed because the profiles aren’t an exact match and may require a bit of PCR optimization to say more conclusively.
I’m again going to quote Chris White (White Labs) on mixed-strain fermentations:
“When yeast is pitched into beer, it starts to grow, entering into a log phase of growth after a few hours. This is when the bulk of the flavor compounds are produced. 12-36 hours into the fermentation. Therefore,
if your goal is flavor, you need to add the multiple strains early on, preferably together. Note that if you just want another strain for bottling, or to complete attenuation, go ahead and add later. Very little flavor contribution happens here, unless the beer undergoes prolonged ageing”
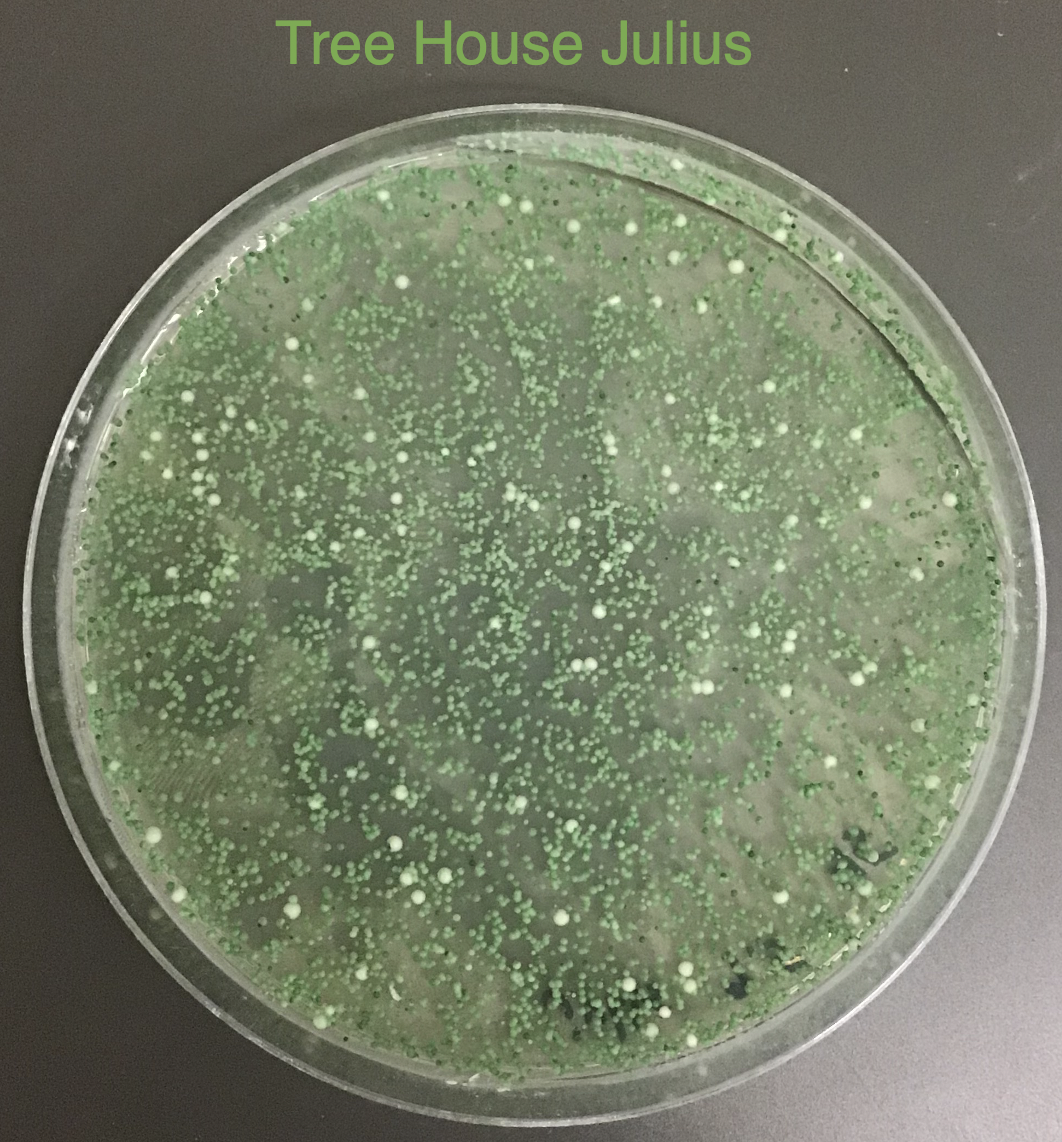
You'll notice that on some of the WLN plates it looks like S-04 (small green colonies) is 99+% of the plate.
I'm pretty near my limit on the amount of time I can spend on this, but because the composition of yeast in the can will change over time (all beers above are typically analyzed at 1-2 months old) I am willing to test a few very fresh cans (ideally <10 days), PM me if you can mail. These will be the most indicative of the ratio of S-04 to everything else at the time of canning.









![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)