You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How do I convert saurmalz to lactic acid?
- Thread starter JHulen
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
JHulen
Well-Known Member
- Joined
- Mar 30, 2019
- Messages
- 53
- Reaction score
- 41
If a recipe calls for 2% of the grist to be acid malt, how would I know how much lactic acid to use?
Sorry, I mean in place of acid malt
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
This is merely ballparking it, but first lets look at 88% lactic acid:
Denisty = 1.209 g./mL
Concentration of lactic acid in 88% lactic acid = 88% (by weight)
1.209 g./mL* 0.88 = 1.06392 grams of pure 100% lactic acid in every mL of 88% lactic acid.
--------------------------------------------------------------------------------------------------------
Now lets look at Acid Malt, for a case where 5 Oz. of it are called for in a recipe to hit a targeted mash pH.
5 ounces * 28.35 grams/Oz. = 141.75 grams
Assume (a ballpark figure of) 3% by weight lactic acid "equivalent" in acid malt, therefore:
141.75 g. x 0.03 = 4.2525 grams of lactic acid are required, as well as "presumed" present within 5 Oz. of acid malt.
4.2525 grams / 1.06392 grams/mL = 3.99701 mL of 88% lactic acid
Answer = 4 mL of 88% lactic acid is going to be close to 5 ounces of acidulated malt or sauermalz
And further:
5 ounces / 3.99701 mL = 1.251 (so each mL of 88% lactic acid is roughly the equivalent of 1.25 ounces of acid malt)
and:
3.99701 mL / 5 ounces = 0.8 (so 1 ounce of acid malt is roughly the equivalent of 0.8 mL of 88% lactic acid)
These are merely ballparks predicated upon the acid found within acid malt being presumed to be roughly equivalent to 3% lactic acid by weight. I would start here and see how it works out. Acid malt is rather variable as to its strength, and it does not correlate directly to lactic acid as presented here, but I would feel comfortable enough to (on first approximation) run with this.
Denisty = 1.209 g./mL
Concentration of lactic acid in 88% lactic acid = 88% (by weight)
1.209 g./mL* 0.88 = 1.06392 grams of pure 100% lactic acid in every mL of 88% lactic acid.
--------------------------------------------------------------------------------------------------------
Now lets look at Acid Malt, for a case where 5 Oz. of it are called for in a recipe to hit a targeted mash pH.
5 ounces * 28.35 grams/Oz. = 141.75 grams
Assume (a ballpark figure of) 3% by weight lactic acid "equivalent" in acid malt, therefore:
141.75 g. x 0.03 = 4.2525 grams of lactic acid are required, as well as "presumed" present within 5 Oz. of acid malt.
4.2525 grams / 1.06392 grams/mL = 3.99701 mL of 88% lactic acid
Answer = 4 mL of 88% lactic acid is going to be close to 5 ounces of acidulated malt or sauermalz
And further:
5 ounces / 3.99701 mL = 1.251 (so each mL of 88% lactic acid is roughly the equivalent of 1.25 ounces of acid malt)
and:
3.99701 mL / 5 ounces = 0.8 (so 1 ounce of acid malt is roughly the equivalent of 0.8 mL of 88% lactic acid)
These are merely ballparks predicated upon the acid found within acid malt being presumed to be roughly equivalent to 3% lactic acid by weight. I would start here and see how it works out. Acid malt is rather variable as to its strength, and it does not correlate directly to lactic acid as presented here, but I would feel comfortable enough to (on first approximation) run with this.
Last edited:
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
If a recipe calls for 2% of the grist to be acid malt, how would I know how much lactic acid to use?
I was just pondering this. If a specific written recipe is calling for 2% of the grist to be sauermalz (acid malt, or acidulated malt) by weight, then the recipe must be strongly presuming that both your water analyticals and your mashes water to grist ratio are identical to those of the recipe builder, and further that your grist components acid/base characteristics are also identical to the grist of the recipe builder. Those are mighty big presumptions, with a strong likelihood for error.
Last edited:
Big Monk
Trappist Please! 🍷
- Joined
- Dec 24, 2015
- Messages
- 2,192
- Reaction score
- 1,151
The short answer is you can’t convert a mass of Sauermalz to a corresponding volume of Lactic Acid. Sauermalz is pale malt, typically Pilsner, sprayed in most cases with biological acid (Sauergut). Sauergut also can’t be modeled as Lactic Acid.
The fact of the matter is that the titration curves for Sauermalz and Lactic Acid only cross and show parity at an extremely small pH range.
Your best bet is to punch the recipe into a pH estimation software and target your desired pH with Lactic Acid.
The fact of the matter is that the titration curves for Sauermalz and Lactic Acid only cross and show parity at an extremely small pH range.
Your best bet is to punch the recipe into a pH estimation software and target your desired pH with Lactic Acid.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
The short answer is you can’t convert a mass of Sauermalz to a corresponding volume of Lactic Acid.
@RPIScotty, are you emphatically stating that even attempting to ballpark a generalized correlation "ratio" is a total waste of time and effort? Further, if any such "ballpark" correlation requires a very narrow pH range, would not the setting of a 5.2-5.6 mash pH target be confining things to a rather narrow pH range?
Big Monk
Trappist Please! 🍷
- Joined
- Dec 24, 2015
- Messages
- 2,192
- Reaction score
- 1,151
@RPIScotty, are you emphatically stating that even attempting to ballpark a generalized correlation "ratio" is a total waste of time and effort? Further, if any such "ballpark" correlation requires a very narrow pH range, would not the setting of a 5.2-5.6 mash pH target be confining things to a rather narrow pH range?
I’d rather not clog up this fellas thread with a back and forth between us. That isn’t what the OP is on about anyway.
Yes. I think historically speaking most people avoid using Sauermalz because of how inconsistent the “Sauermalz by weight of Lactic Acid” system is. Part of the inconsistency is that Sauermalz isn’t Lactic Acid and can’t be modeled as such.
Back on topic. To the OP: if you know the desired pH of the recipe, replace the Sauermalz with base malt and use an amount of Lactic Acid to hit that pH.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
This web page from Kai Troester (Braukaiser) indicates that 0.085 mL of 88% lactic acid, and a nominal 3 grams of the specific lots of acid malt which he tested both contain 1 mEq of acid (see chart about 1/6 of the way down the page).
http://braukaiser.com/wiki/index.php/Mash_pH_control
3 grams / 28.35 = 0.10582 ounces of acid malt
0.10582 ounces acid malt /0.085 mL of 88% lactic acid = 1.245 (meaning that 1.245 ounces of acid malt is the acid equivalent of 1 mL of 88% lactic acid)
The inverse of 1.245 is 0.8032 (meaning that 0.8032 mL of 88% lactic acid is the acid equivalent of 1 ounce of acid malt)
These Braukaiser ratios of acid equivalence (which I presume he derived from actual testing) are for all practical purposes identical to the ratios which I derived above simply by presuming a ballpark of 3% lactic acid "equivalent" in a typical lot of acid malt.
http://braukaiser.com/wiki/index.php/Mash_pH_control
3 grams / 28.35 = 0.10582 ounces of acid malt
0.10582 ounces acid malt /0.085 mL of 88% lactic acid = 1.245 (meaning that 1.245 ounces of acid malt is the acid equivalent of 1 mL of 88% lactic acid)
The inverse of 1.245 is 0.8032 (meaning that 0.8032 mL of 88% lactic acid is the acid equivalent of 1 ounce of acid malt)
These Braukaiser ratios of acid equivalence (which I presume he derived from actual testing) are for all practical purposes identical to the ratios which I derived above simply by presuming a ballpark of 3% lactic acid "equivalent" in a typical lot of acid malt.
Last edited:
Interesting stuff guys . I do appreciate you science guys . This topic makes me wonder about phosphoric acid vs Lactic acid . If a recipe calls for 1 tsp of phosphoric can you use the same amount of Lactic instead ?
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Interesting stuff guys . I do appreciate you science guys . This topic makes me wonder about phosphoric acid vs Lactic acid . If a recipe calls for 1 tsp of phosphoric can you use the same amount of Lactic instead ?
Off the top of my head 88% lactic acid is ballpark 1/10.5 (give or take, since I'm pulling this off the top of my head) by volume to 10% Phosphoric Acid. So it would be closer to 1/10 TSP of 88% lactic acid being the ballpark equivalent of 1 TSP of 10% phosphoric acid.
Big Monk
Trappist Please! 🍷
- Joined
- Dec 24, 2015
- Messages
- 2,192
- Reaction score
- 1,151
@JHulen , I apologize for the hijacking that is about to happen here, but at least I think we have answered your question in a number of ways, so that's a plus.
A few things:
1.) Sauermalz, aka Acidulated Malt is just that: Malt. The best way to model it is as a malt, i.e. using titration parameters. In fact, the best luck I have ever had with it has been after switching to the pH DI, a1, a2, and a3 Q equation. No more strength modifiers, no more guessing what % of Lactic it is.
2.) Sauermalz, AFAIK based on academic texts and industry papers is NOT treated with straight Lactic Acid, but rather Sauergut (Biological Acid) at a much lower acid %. To further complicate things, you also can NOT treat Sauergut as an equivalent to straight Lactic Acid.
I always go back here:
https://www.homebrewtalk.com/forum/...z-of-a-typical-acid-malt.662019/#post-8510857
So in fairness, even a blind squirrel finds a nut every now and then and I guess having an approximation that relates Sauermalz by weight to an equivalent in 88% Lactic Acid is some form a primitive blind squirrel nut radar.
The problem is you won't know exactly what pH your mash will settle to and if you estimate the Sauermalz weight based on one value and your mash settles at 5.2, the acid characteristics of the addition could skew things. Then you are messing with acid percentages of Sauermalz or Strength modifiers and chasing your tail.
Full disclosure: I am a proponent of the proton deficit model so my biased opinion is that modeling Sauermalz as a malt using the following:
Q (mEq) Sauermalz = kg * (a1 * (pHz - pHDI) + a2 * (pHz - pHDI) ^2 + a3 * (pHz - pHDI) ^3)
is the best way to estimate the acid characteristics of Sauermalz.
This has worked best for me but also has it's drawbacks, namely the fact that you need all the titration information to use it.
Fortunately, however, I have confidence in the known characteristics for a few reasons:
1.) The deLange and Walts data show relative parity across pH DI, a1, a2, and a3;
2.) I've got 4 years of Weyermann Malt Analysis data showing Sauermalz pH DI as rock solid for that period as well as acid content.
The verdict?
Why does the titration information seem rock solid while conventional wisdom says that there is a ton of variance in Sauermalz?
This is likely due to some of the technical stuff shown above, i.e. assuming you can model Sauermalz by weight as an equivalent to 88% Lactic Acid (at static pHz) whereas titration takes pHz into account dynamically, i.e. Q (mEq) changes with pHz.
Just my $0.02...
A few things:
1.) Sauermalz, aka Acidulated Malt is just that: Malt. The best way to model it is as a malt, i.e. using titration parameters. In fact, the best luck I have ever had with it has been after switching to the pH DI, a1, a2, and a3 Q equation. No more strength modifiers, no more guessing what % of Lactic it is.
2.) Sauermalz, AFAIK based on academic texts and industry papers is NOT treated with straight Lactic Acid, but rather Sauergut (Biological Acid) at a much lower acid %. To further complicate things, you also can NOT treat Sauergut as an equivalent to straight Lactic Acid.
I always go back here:
https://www.homebrewtalk.com/forum/...z-of-a-typical-acid-malt.662019/#post-8510857
Kai understands the chemistry so when he says the acid equivalence is 3.5% he is talking about approximate acid equivalence in a narrow range of pH.
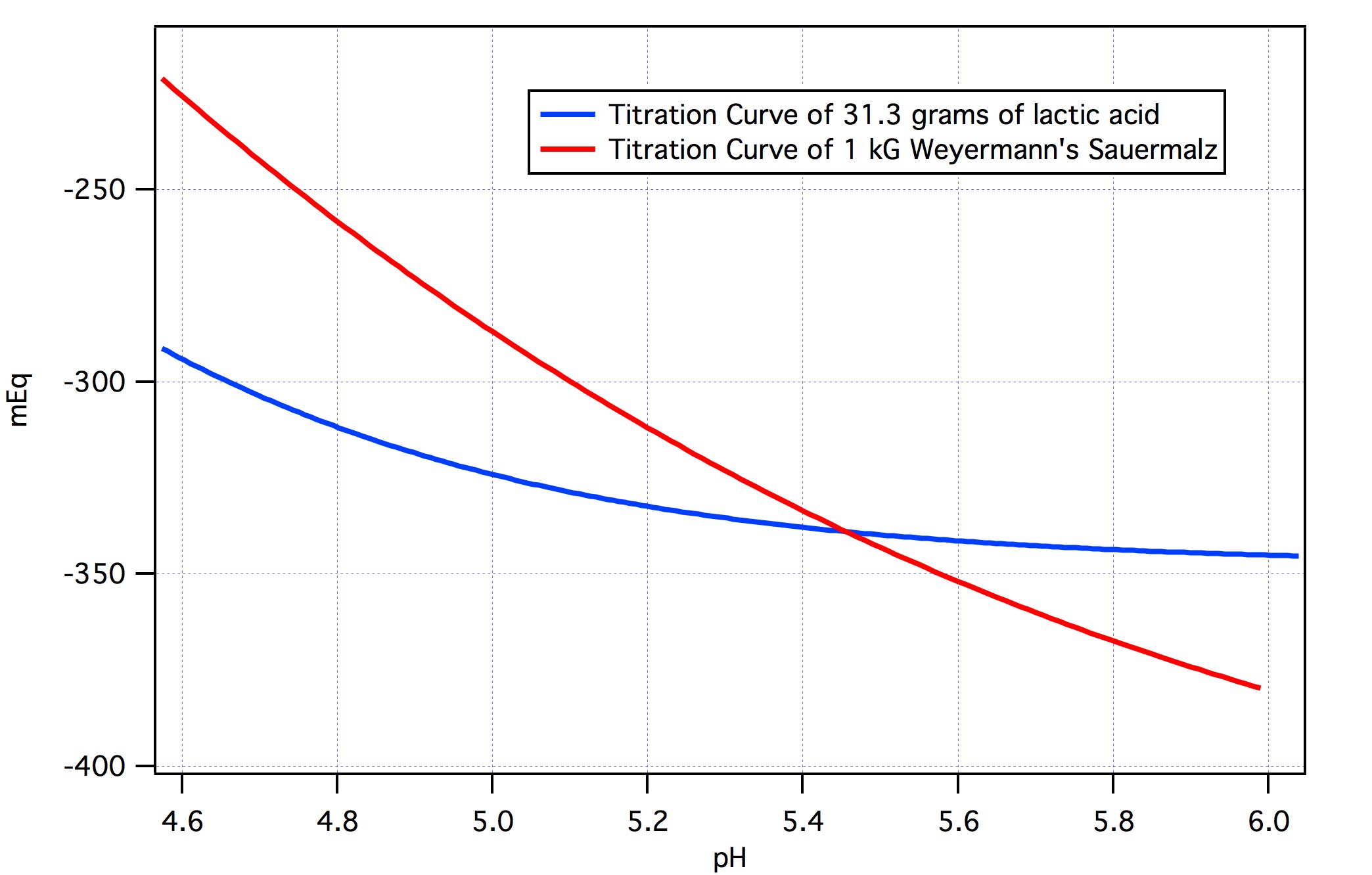
What you would have to do is plot out the titration curve of Sauermalz and the titration curve of Lactic Acid and then scale the Lactic Acid curve to come as close as possible to the Sauermalz curve over some range of interest (presumably the range of desired mash pH's). The curves below do this for 1 kg of Weyermann's Sauermalz and 31.3 grams of lactic acid.
The fit was done over the region pH 4.6 to 6 and, as the graph shows, the match is perfect at about 5.45. In other words, 31.3 grams of lactic acid are equivalent in acidity to 1 kG of Weyermann's Sauermalz at pH 5.45. At any other pH this equivalent does not hold. Thus at pH 5.45 Weyermann's Sauermalz has an effective lactic acid content of 3.13%.
So in fairness, even a blind squirrel finds a nut every now and then and I guess having an approximation that relates Sauermalz by weight to an equivalent in 88% Lactic Acid is some form a primitive blind squirrel nut radar.
The problem is you won't know exactly what pH your mash will settle to and if you estimate the Sauermalz weight based on one value and your mash settles at 5.2, the acid characteristics of the addition could skew things. Then you are messing with acid percentages of Sauermalz or Strength modifiers and chasing your tail.
Full disclosure: I am a proponent of the proton deficit model so my biased opinion is that modeling Sauermalz as a malt using the following:
Q (mEq) Sauermalz = kg * (a1 * (pHz - pHDI) + a2 * (pHz - pHDI) ^2 + a3 * (pHz - pHDI) ^3)
is the best way to estimate the acid characteristics of Sauermalz.
This has worked best for me but also has it's drawbacks, namely the fact that you need all the titration information to use it.
Fortunately, however, I have confidence in the known characteristics for a few reasons:
1.) The deLange and Walts data show relative parity across pH DI, a1, a2, and a3;
2.) I've got 4 years of Weyermann Malt Analysis data showing Sauermalz pH DI as rock solid for that period as well as acid content.
The verdict?
Why does the titration information seem rock solid while conventional wisdom says that there is a ton of variance in Sauermalz?
This is likely due to some of the technical stuff shown above, i.e. assuming you can model Sauermalz by weight as an equivalent to 88% Lactic Acid (at static pHz) whereas titration takes pHz into account dynamically, i.e. Q (mEq) changes with pHz.
Just my $0.02...
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Even Weyermann itself admits that the acid found in their acidulated malt is precisely lactic acid, albeit derived (as correctly stated by RPIScotty above) via natural bacterial action.
https://www.weyermann.de/cz/faq.asp?umenue=yes&idmenue=33&sprache=1
Likely the main reason why their mEq/pH acidity curves deviate a bit is that acid malt has the basic (with respect to a typical 5.4 mash pH target) component of the very base malt that it is combined with inseparably intermingled with it, so there are two competing factions of acidity (the base malts low acidity [basic with respect to 5.4 pH] combined with the naturally derived lactic acids acidity).
Weyermann® Acidulated Malt is produced by using lactic acid, which is generated by on grain natural occurring lactic bacteria.
https://www.weyermann.de/cz/faq.asp?umenue=yes&idmenue=33&sprache=1
Likely the main reason why their mEq/pH acidity curves deviate a bit is that acid malt has the basic (with respect to a typical 5.4 mash pH target) component of the very base malt that it is combined with inseparably intermingled with it, so there are two competing factions of acidity (the base malts low acidity [basic with respect to 5.4 pH] combined with the naturally derived lactic acids acidity).
Last edited:
Big Monk
Trappist Please! 🍷
- Joined
- Dec 24, 2015
- Messages
- 2,192
- Reaction score
- 1,151
Even Weyermann itself admits that the acid found in their acidulated malt is precisely lactic acid, albeit derived (as correctly stated by RPIScotty above) via natural bacterial action.
https://www.weyermann.de/cz/faq.asp?umenue=yes&idmenue=33&sprache=1
Likely the main reason why their mEq/pH acidity curves deviate a bit is that acid malt has the basic (with respect to a typical 5.4 mash pH target) component of the very base malt that it is combined with inseparably intermingled with it, so there are two competing factions of acidity (the base malts acidity low acidity [basic with respect to 5.4 pH] combined with the lactic acids acidity).
The real point is that calculating the acidity of Sauermalz based on a Lactic Acid equivalent is only valid at a specific pHz value. It's static because changes in pHz don't then change the amount of Sauermalz accordingly to compensate.
Calculating Sauermalz as a malt with titration parameters is dynamic, as changes in pHz change the Q value.
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
Scanning the AJ diagram for a collection of data points, and seeing where quite fortunately lactic acids mEq's remain relatively stable (or flat) down to pH 5.4 and then continues to remain decently stable (flat) down to pH 5.1, I utilized the data from the diagram to model a third order quadratic equation based multiplier/corrector which mirrors the charts differential between lactic acid and acid malt with changing pH, and now permits me to more accurately determine correct acid malt addition quantities based upon (or in relation to) lactic acid (with the presumption that the diagram itself is correct). But whether or not this pH variable (dynamic) multiplier/corrector is applied to the static method I initially used above the alteration/correction introduced by its induced correction does not appear to me great enough (in my opinion) to stop anyone from benefiting from going ahead and using the simple method I outlined in post #3, and which seems to be supported by Braukaiser (as seen in post #8). This third order quadratic based correction factor for acid malt will appear in MME version 6.45. I'm testing both versions (standard and metric) presently.
Last edited:
Big Monk
Trappist Please! 🍷
- Joined
- Dec 24, 2015
- Messages
- 2,192
- Reaction score
- 1,151
Scanning the AJ diagram for a collection of data points, and seeing where quite fortunately lactic acids mEq's remain relatively stable (or flat) down to pH 5.4 and then continue to remain decently stable (flat) down to pH 5.1, I utilized the data from the diagram to model a third order quadratic equation based multiplier/corrector which mirrors the charts differential between lactic acid and acid malt with changing pH, and now permits me to more accurately determine correct acid malt addition quantities based upon (or in relation to) lactic acid (and of course with the presumption that the diagram itself is correct). But even if this pH variable (dynamic) multiplier/corrector is not applied to the static method I initially used above the alteration/correction introduced by it does not appear to me great enough (in my opinion) to stop anyone from benefiting from going ahead and using the simple method I outlined in post #3, and which seems to be supported by Braukaiser (as seen in post #8). This third order quadratic based correction factor for acid malt will appear in MME version 6.45. I'm testing both versions (standard and metric) presently.
The proof is in the pudding. The Sauermalz as equivalent Lactic Acid method is known to be flawed. For every situation that requires a band-aid, there is a situation that will cut you and require another band-aid.
Do what works for you but don't keep revising your sheet to accommodate things you haven't tested. I have tons of data points from my collaborator and myself that shows the Sauermalz as equivalent Lactic Acid method is highly dependent on specific mash conditions and the strength modifiers/Acid % numbers you feed it. That's not sustainable.
I try to trial 2-3 batches on new calculations before I issue anything. YMMV.
If you do the same thing you've always done you'll get the same results you've always gotten. A very sharp homebrewer once said that...
Silver_Is_Money
Larry Sayre, Developer of 'Mash Made Easy'
The AJ diagram shows us that the greatest differential in mEq's between the two forms of acid within the pH ranges of importance to acid addition is only on the order of about 7%. So the required correction to a static model is at most going to be on the order of 7%. I agree that buffering values related to ones aggregate grist make-up will also play a part.
I use sour malt. I have no idea how to convert sour malt volume into lactic acid volume. It would be difficult to do because the pH of sour malt varies.
I think it's good to know what the inherent pH of the base malt is before making pH adjustments. The pH is usually indicated on the spec sheet that comes with every bag of malt.
During the acid rest mash pH reduces. The longer the rest the lower the pH. To reduce time rest the mash for an hour at 95F, check pH, add sour malt or lactic acid, if necessary. The short rest will allow the inherent pH of the malt to reduce mash pH. Mash pH should be established before enzymes activate.
With single temperature brewing methods using temperatures above 145F mash pH should favor Alpha because the enzyme is responsible for liquefaction and saccharification. When a conversion rest is used pH is adjusted to favor Beta.
Whether, the choice is sour malt or lactic acid, a good pH meter is required. With sour malt, patience is needed because the stuff works slow and it's easy to go over board with the malt. From the taste aspect, the taste imparted by sour malt is smoother and not as sharp as the taste from lactic acid. The taste of sour malt varies. For brewers that use sour malt Meussdoerffer sour malt is exceptional sour malt, but, it's pricey.
I think it's good to know what the inherent pH of the base malt is before making pH adjustments. The pH is usually indicated on the spec sheet that comes with every bag of malt.
During the acid rest mash pH reduces. The longer the rest the lower the pH. To reduce time rest the mash for an hour at 95F, check pH, add sour malt or lactic acid, if necessary. The short rest will allow the inherent pH of the malt to reduce mash pH. Mash pH should be established before enzymes activate.
With single temperature brewing methods using temperatures above 145F mash pH should favor Alpha because the enzyme is responsible for liquefaction and saccharification. When a conversion rest is used pH is adjusted to favor Beta.
Whether, the choice is sour malt or lactic acid, a good pH meter is required. With sour malt, patience is needed because the stuff works slow and it's easy to go over board with the malt. From the taste aspect, the taste imparted by sour malt is smoother and not as sharp as the taste from lactic acid. The taste of sour malt varies. For brewers that use sour malt Meussdoerffer sour malt is exceptional sour malt, but, it's pricey.
Similar threads
- Replies
- 8
- Views
- 331
- Replies
- 9
- Views
- 584
- Replies
- 13
- Views
- 3K
- Replies
- 0
- Views
- 408

