SHAIV
Well-Known Member
- Joined
- Sep 8, 2015
- Messages
- 615
- Reaction score
- 160
Hey so ive been researching water profiles and adjusting water. My last beer i brewed i started with distilled and built my water from that with brewers friend software. Ill probably use bru’n water next time though. Its more detailed. Anyway im interested in skipping the step of going to walmart to get distilled water and using my home water for a source. Ive got a carbon filter for a refrigerator that i could probably rig up under my sink and use it strictly fir brew water. Or i have a friend that will give me an out of service RO filter system that i might could use for brewing. I dont really want to fork over 42$ for ward labs test at this time. I know, im cheap lol. If i have to i may end up doing that eventually but not right now. I emailed my city water supply and i got some info on my water. It seems a harsh to drink to me so ive never used it for brewing before. I know i would have to get rid of the chlorine first at least but i got this info on my water. I believe i got a few water inputs but i may be missing a few. Heres the info i got

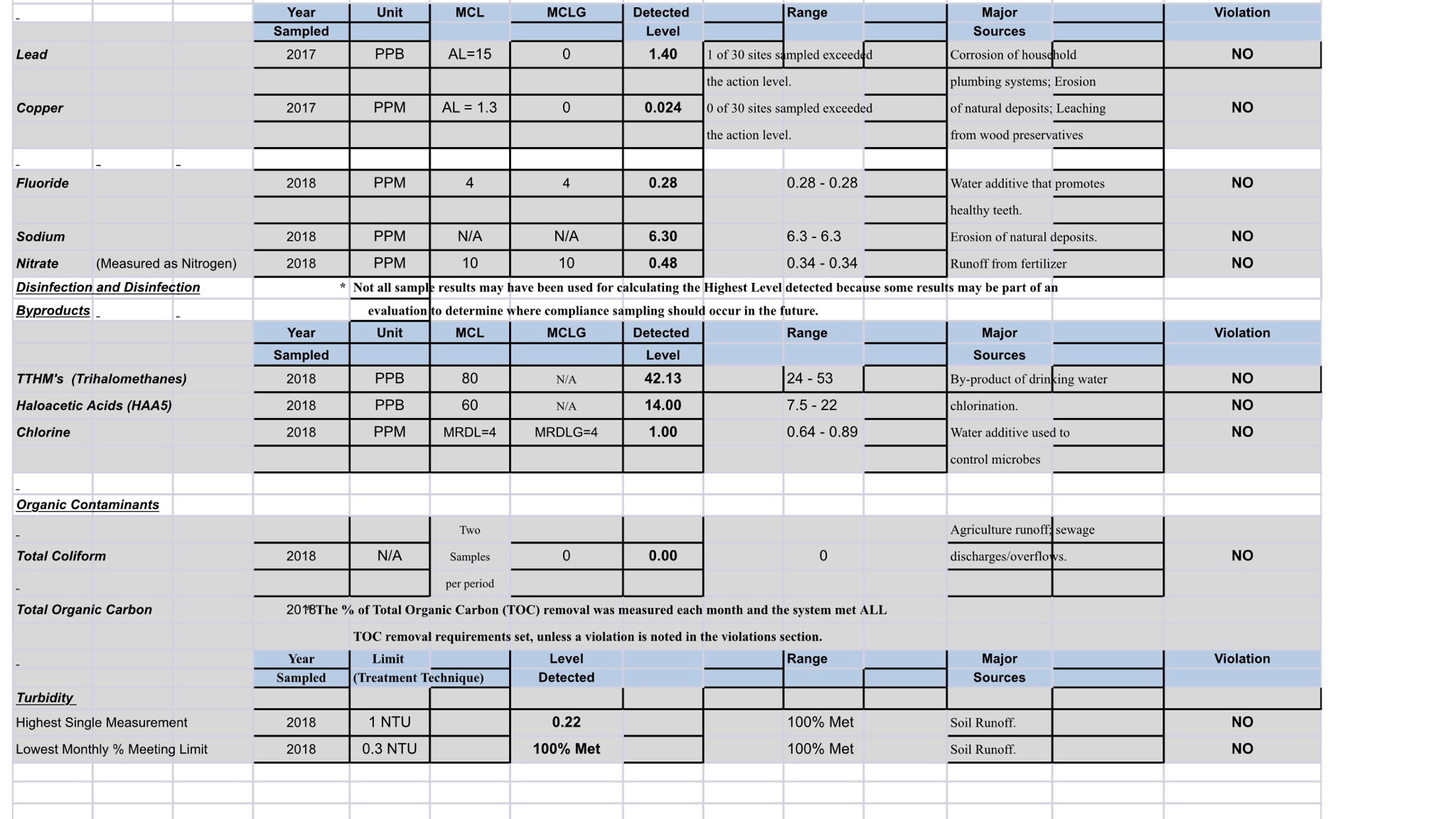
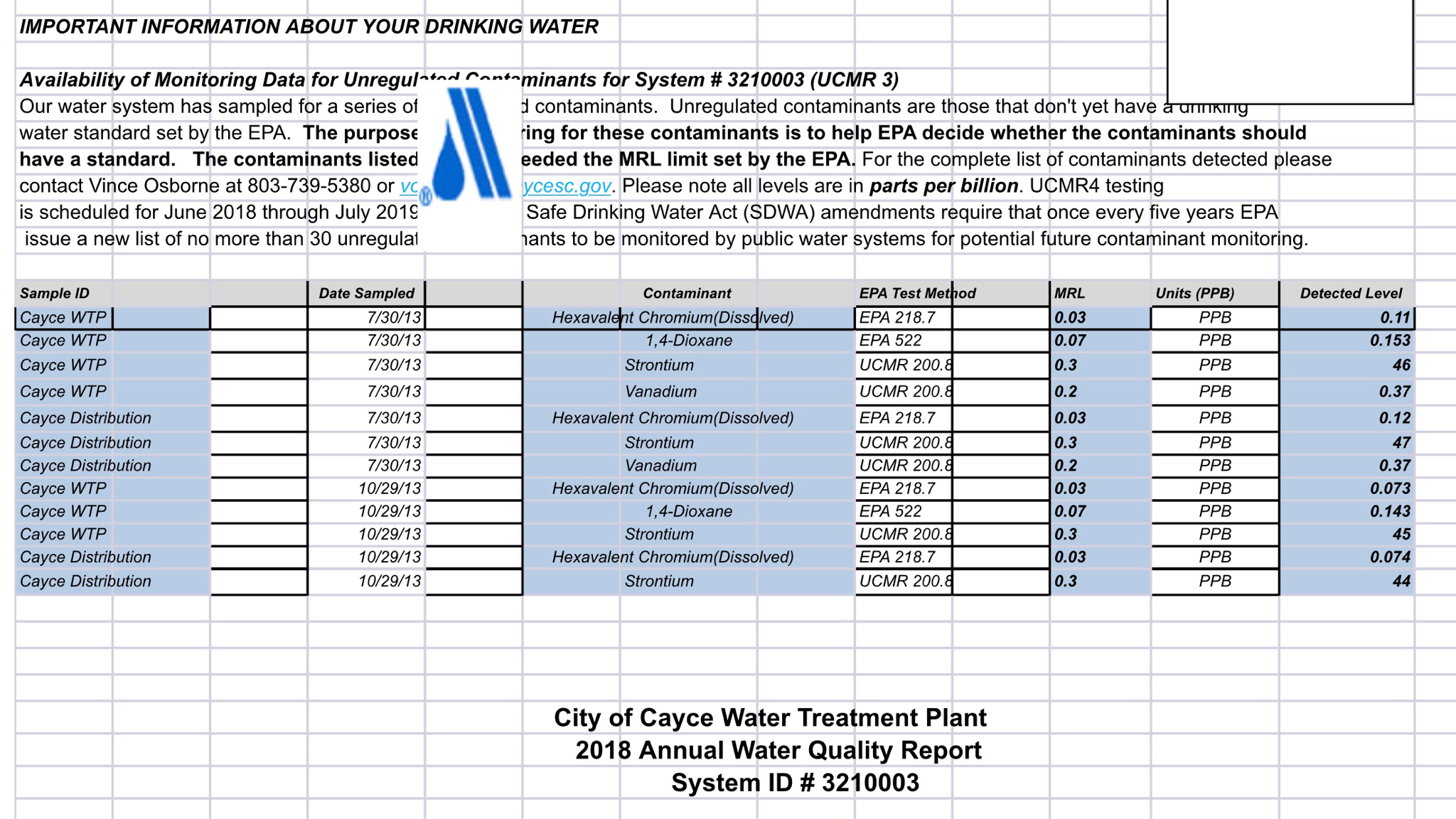
I think i have PH, calcium, sodium, and maybe one more.
Is there anything im missing? Is there something here that is renamed or something? Like hardness is calcium right? Or wrong?



I think i have PH, calcium, sodium, and maybe one more.
Is there anything im missing? Is there something here that is renamed or something? Like hardness is calcium right? Or wrong?

















































![Craft A Brew - Safale BE-256 Yeast - Fermentis - Belgian Ale Dry Yeast - For Belgian & Strong Ales - Ingredients for Home Brewing - Beer Making Supplies - [3 Pack]](https://m.media-amazon.com/images/I/51bcKEwQmWL._SL500_.jpg)