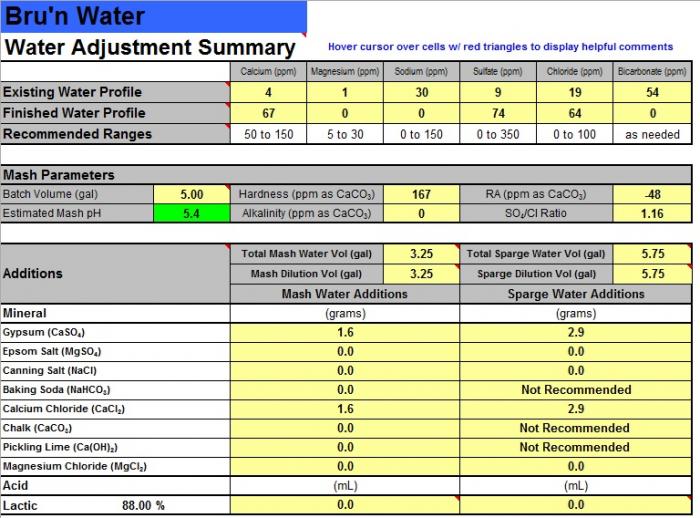
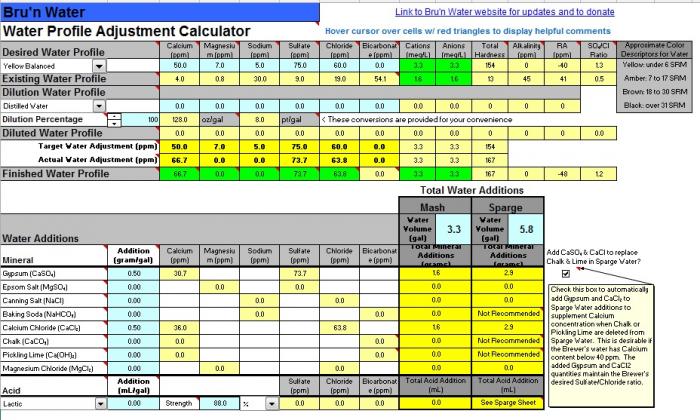
I'm trying to improve my process and a big part of that is measuring. The mash seems like an important part of my process that is ripe for improvement so I've been reading up on water chemistry and the mash process. I had not appreciated the importance of pH and I'm trying to implement what I've learned. From what I've gathered, I need to hit a particular pH target range during the mash and should also make sure not to drain beyond when the runnings hit a particular pH and OG to avoid extracing tannins or whatever.
But taking all these measurements seems like a real PITA, especially since I've read that pH readings should be taken at room temp. Last weekend, after letting the mash sit for 5 minutes, I pulled a sample, cooled it and tested it. It was 4.6. So I added chalk and waited another five minutes, pulled a sample, tested, and it was still a little low (maybe 5.0? I don't know if I'm color blind or what but I'm not very good at matching colors). So I added another 1/2 tsp of chalk. By that point I was probably 20 minutes into my mash and figured it should be close enough. But that's a lot of mash time at the wrong pH.
Then when it comes time to collect the runnings, do you just stop draining when the flow slows down and take 5+ minutes to check the pH and gravity? Then if its still ok, drain again, stop again, check again? I typically collect three separate runnings so this seems really inefficient.
I'm wondering whether people who are mindful of the mash pH have any ideas on how to improve this process or whether they take all of these measurements.
But taking all these measurements seems like a real PITA, especially since I've read that pH readings should be taken at room temp. Last weekend, after letting the mash sit for 5 minutes, I pulled a sample, cooled it and tested it. It was 4.6. So I added chalk and waited another five minutes, pulled a sample, tested, and it was still a little low (maybe 5.0? I don't know if I'm color blind or what but I'm not very good at matching colors). So I added another 1/2 tsp of chalk. By that point I was probably 20 minutes into my mash and figured it should be close enough. But that's a lot of mash time at the wrong pH.
Then when it comes time to collect the runnings, do you just stop draining when the flow slows down and take 5+ minutes to check the pH and gravity? Then if its still ok, drain again, stop again, check again? I typically collect three separate runnings so this seems really inefficient.
I'm wondering whether people who are mindful of the mash pH have any ideas on how to improve this process or whether they take all of these measurements.